Contents |
Take-Home Message
A primary function of the energy systems we use to formulate diets is to value different feeds accurately. If 1 gram of sawdust is burned, it will release about the same amount of heat as if 1 gram of corn grain was burned, but if fed to a cow, sawdust will support substantially less milk production than corn. Significant and highly variable losses of energy occur as a feed or diet is digested and metabolized. If we can accurately account for those losses, we can accurately evaluate different feedstuffs. Energy can be lost from an animal in the form of chemical energy (feces, urine, and methane) and in the form of heat. Digestible energy accounts for only fecal loss which is usually the largest and most variable loss, but it does not adequately differentiate feeds. Metabolizable energy (ME) accounts for energy lost via feces, urine, and methane and is more accurate than digestible energy, but it does not account for the second most variable energetic loss (heat). Net energy (NE) accounts for all losses and theoretically is the most accurate method for differentiating feeds. The NE systems is based on the first law of thermodynamics (i.e., energy cannot be destroyed or created). If we can accurately estimate the NE of a diet and we know NE requirements, then energy balance is also known. Because the NE concentration of a diet can only be measured using a very expensive calorimeter, NE values used to formulate diets are only estimates, and substantial errors can exist in the estimates. The NE requirements are known with greater accuracy than NE values of diets, but some errors can occur. Because of the errors in estimating NE requirements and especially NE supply, the practicing nutritionist must make adjustments based on estimated energy balance (change in body condition).
Please check this link first if you are interested in organic or specialty dairy production
Introduction
Antoine Lavoisier, a French chemist, and to a lesser extent, Joseph Priestly, a British philosopher, are considered the founding fathers of bioenergetics (they also were co-discoverers of oxygen). Their studies in the late 18th century proved that animals “combust” food. Lavoisier in the late 1700s designed and built an animal calorimeter (it housed a guinea pig) and determined that an animal and a fire produced the same amount of heat per unit of carbon dioxide produced (Kleiber, 1975). Others showed that the amount of heat produced when an animal metabolized a substance was the same as the amount of heat produced when that substance was burned. Once animal metabolism was equated to a flame, thermodynamic principles developed by physicists and engineers (mainly of steam engines) began to be applied to animal nutrition. Nutritionists, although they may not realize it, use the two fundamental laws of thermodynamics (plus portions of Einstein’s theory of relativity) every time they evaluate the energy nutrition of an animal.
Laws of Thermodynamics
The first law of thermodynamics is: Energy cannot be created or destroyed (i.e., it must be conserved).
The second law of thermodynamics is: Entropy of the universe always increases.
These two seemingly abstract statements are the foundation of the net energy systems we use to formulate diets and evaluate energy status of animals. In addition to those laws, bioenergetics is also based on Einstein’s theory of relativity, i.e., the equivalence between mass and energy. In other words, we can convert measures of mass (pounds) to measures of energy (calories).
In terms relevant to animal nutrition, the first law of thermodynamics can be stated as: Energy input must equal energy output plus or minus any change in body energy. Although energy can enter an animal’s body via radiation (e.g., direct exposure to the sun’s rays), convection (flow of heat from the air to the body), and conductance (flow of heat from a warm surface that is in contact with the animal), these inputs are usually quite minor (for example, convection can only occur when environmental temperature exceeds body temperature, which is not very common). Therefore, this discussion will assume the only energy input into the body is via diet. The main ways energy can leave an animal is via feces, urine, gas (predominantly methane), milk, and heat. A small loss of mass (i.e., energy) occurs via the body surface (hair, skin cells, body secretions containing carbon, etc.), but these losses are usually quite small (<0.5% of energy consumed) and will be ignored in this discussion. In most situations, the first law of thermodynamics in animal nutrition terms can be written as:
Body Energy Change = IE – FE – UE – GE – MiE – Heat
where: IE = intake energy (E), FE = fecal E, UE = urine E, GE = gas E, MiE = milk E
Although this equation is applicable from the point of conception, the starting point for this discussion will be birth. An average newborn calf that weighs 45 kg (100 lb) has a total body energy content of about 58 Mcal (mass and energy are interchangeable; therefore, a newborn infant can be considered as an 8-pound bundle of joy or a 7000-kcal bundle of joy). When the calf reaches maturity and weighs 1400 lb, she will have a total body energy content of about 1800 Mcal. Based on the first law of thermodynamics, all energy consumed by that animal as it grew must be accounted for (Figure 1).
The second law of thermodynamics is also illustrated in Figure 1. The second law can be paraphrased as: no transformation of energy is 100% efficient and the inefficiencies are lost as heat. Examples of energy transformations performed by an animal are: 1) chemical energy of food converted into muscle, 2) chemical energy of a food converted into physical activity (e.g., breathing or walking), and 3) chemical energy of body fat converted into milk. When each of these transformations are done, part of the energy input is converted to the energy output, and part is converted to heat. The synthesis of lactose from propionic acid by a dairy cow illustrates this principle. If the cow makes lactose only from propionic acid, it takes 4.7 molecules of propionic
acid to make 1 molecule of lactose. If 4.7 moles of propionic acid are burned completely, it releases 1725 kcal of heat and 1 mole of lactose; if completely combusted, it releases 1350 kcal of heat. The cow took 1725 kcal of chemical energy (propionic acid) and converted it into 1350 kcal of chemical energy (lactose), which means that 375 kcal (1725 – 1350) were lost, and that loss was heat. The efficiency of that reaction was 1350/1725 = 0.78. The conversion of chemical energy into mechanical energy in an animal results in essentially all the chemical energy being converted into heat even though a mechanical process has occurred. The reason for this is when a muscle fiber contracts to do mechanical work, it returns to its original place (i.e., no net change in distance has occurred), and therefore no “true work” has been conducted (even though the animal may have walked a mile, the part of the muscle fiber that moves during contraction has not moved relative to the other part of the muscle fiber). Therefore, if an animal expends 1 Mcal of chemical energy from the diet to walk, breathe, pump blood, etc., that 1 Mcal of chemical energy is lost from the body as heat. The massive amount of heat lost (~10,000 Mcal or more than 26 million BTUs) as the cow (Figure 1) grew from birth to maturity is a result of the inefficiencies of converting one form of chemical energy into another form and from the physical activity associated with life.
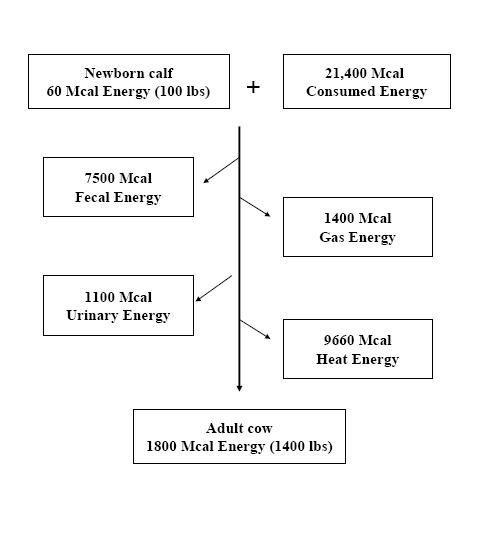
Definitions of Energy Flow in Animals
In the U.S., the calorie is the energy unit usually used in applied human and animal nutrition (in this paper, Mcal will be the standard unit [(1 Mcal = 1 million calories or 1000 kcal]). In scientific journals and in several other countries, the Joule is used to express energy. Because this paper is for practicing nutritionists, calories will be used, but mega Joules (MJ) and megacalories are interchangeable (1 MJ = 0.239 Mcal; 1 Mcal = 4.184 MJ).
Intake or gross energy (GE) is the total amount of chemical energy in the diet (expressed as Mcal/kg or Mcal/lb of diet dry matter) or the total amount of energy consumed (dry matter intake times energy concentration, expressed as Mcal/day). Energy concentration is measured by completely burning a sample of the diet and measuring the heat produced using a bomb calorimeter. This measurement is easy, precise, and accurate. The concentration of GE in a diet (or feed) depends solely on the chemical composition of the feed. Ash, protein, carbohydrate, and fat have different concentrations of energy per unit of mass; as the concentrations of these fractions change, GE concentration also changes (Table 1). Because organic acids (e.g., lactic acid) have less energy per gram than carbohydrates such as glucose or starch, silages, on average, have less GE than hay or fresh forage. High-protein and high-fat feeds will have more energy than high-carbohydrate feeds, and feeds with high ash will have less energy than lower ash feeds.
| Feed or Compound | Heat of Combustion | |
|---|---|---|
| kcal/g | Mcal/lb | |
| Crude carbohydrate (average) | 4.2 | 1.9 |
| Crude protein (average) | 5.6 | 2.5 |
| Crude fat (average) | 9.4 | 4.3 |
| Ash | 0.0 | 0.0 |
| Glucose | 3.76 | 1.71 |
| Starch and cellulose | 4.23 | 1.92 |
| Leucine | 6.51 | 2.96 |
| Alanine | 4.35 | 1.98 |
| Casein | 5.86 | 2.66 |
| Globulin (a wheat protein) | 5.36 | 2.43 |
| Palmitic acid | 9.35 | 4.25 |
| Soy oil | 9.33 | 4.24 |
| Acetic acid | 3.49 | 1.59 |
| Corn grain | 4.43 | 2.01 |
| Alfalfa hay | 4.37 | 1.99 |
| Soybeans | 5.52 | 2.51 |
Digestible energy (DE) is the energy remaining in the diet after fecal energy is subtracted (Table 2). Daily fecal output (kg/day) is measured, sampled, and burned in a calorimeter. Total fecal energy output (Mcal/day) is subtracted from intake energy (Mcal/day) and then divided by dry matter intake to yield DE as Mcal/kg of diet. Because measurement of DE requires measurement of fecal output, it is less accurate and less precise than measuring GE and can only be measured by feeding animals. Any DE value obtained from a commercial lab is estimated, not measured. The concentration of DE in a feed is a function of GE and all factors (animal and feed) that affect digestibility. The digestibility of the carbohydrate fraction of diets is extremely variable, and much of that variation is caused by the relative concentrations of fiber (less digestible) and nonfiber carbohydrates (more digestible). Chemical (mostly lignin) and physical characteristics of the fiber, and to a lesser extent starch, also influence digestibility. On average, variability in digestibility of the protein and fat fractions is much less than that of the carbohydrate fraction. The major animal factor (besides, digestibility of the carbohydrate fraction of diets is extremely variable, and much of that variation is species-specific, specifically ruminant versus nonruminant) that influences energy digestibility is dry matter intake. The marginal efficiency of digestion decreases as dry matter intake increases.
| Dry Cow | Lactating Cow | |
|---|---|---|
| Dry matter intake, lb/day | 14.3 | 50.2 |
| Energy intake (GE), Mcal/day | 29.6 | 101.6 |
| Fecal energy, Mcal/day | 8.5 (28.7%)1 | 31.1 (30.6%) |
| Urine energy, Mcal/day | 1.3 (4.2%) | 4.0 (3.9%) |
| Methane energy, Mcal/day | 2.5 (8.6%) | 5.8 (5.7% |
| Milk energy, Mcal/day | 0 | 22.7 (37.5%) |
| Heat production, Mcal/day | 17.2 (58.2%) | 34.6 (34.1%) |
| Tissue energy, Mcal/day | 0.1 (0.4%) | 3.3 (6.1% |
| Gross energy, Mcal/day | 2.04 | 2.03 |
| Digestible energy (DE), Mcal/day | 1.45 | 1.41 |
| Metabolizable energy (ME), Mcal/day | 1.19 | 1.21 |
| Net energy (NE), Mcal/day | 0.64 | 0.72 |
| 1Number in parenthesis is percent of energy intake. | ||
Metabolizable energy (ME) is the energy remaining after urinary and gaseous energy arising from fermentation in the digestive tract (essentially methane) is subtracted from DE (Table 2). For nonruminants, gaseous energy is usually quite small and is ignored. Daily urine output is measured and sampled, and its energy concentration is measured by bomb calorimetry to determine total urinary energy lost. For ruminants, either a breathing mask is used, or animals are placed in a chamber so that expelled air can be sampled and methane measured. The amount of methane expelled per day is measured and multiplied by its energy concentration (13.2 Mcal/kg). Daily urine energy and daily methane energy are subtracted from DE and divided by daily dry matter intake to yield ME as Mcal/kg of diet. Collection of urine, bomb calorimetry of urine, and measuring methane are difficult and prone to errors. In addition to those errors, measurement of ME includes all the errors associated with measuring DE; therefore, ME is less accurate and less precise than DE. Measurement of ME requires that an animal be fed the test diet. Any ME provided by a commercial lab is an estimated value. In addition to the factors that affect DE, ME is affected by fiber and protein concentration of the diet. Diets with higher concentrations of fiber alter ruminal (and probably intestinal) bacterial populations so that more acetate and methane are produced. Diets with high protein increase synthesis of urea which is excreted via urine, thereby increasing loss of urinary energy. High-starch diets reduce methane production and therefore increase the efficiency of converting DE to ME. Ionophores such as monensin increase efficiency in part by reducing methane production and increasing the efficiency of converting DE to ME. Animal factors also affect the efficiency of converting DE to ME. High feed intake reduces digestive efficiency, but because high intake has a greater negative effect on fiber digestion than on digestibility of other feed fractions, high intake tends to increase the efficiency of converting DE to ME.
Net energy (NE) is ME minus heat generated by the inefficiency of transforming energy from one form to another (Table 2). That heat is called “heat increment” and is not the same as total heat production. Measuring heat production by an animal requires a whole animal calorimeter. Whole animal calorimeters that can be used with large agricultural animals are extremely expensive, and very few are available in the entire world. Because of the expense and number of facilities, measured NE data are extremely limited. Most calorimeters used for large animals are indirect calorimeters. Oxygen consumption and carbon dioxide production are measured over a period of days, and standard equations are used to convert that information into heat production. For smaller animals, direct calorimetry is often used, which means that actual heat production is measured. Total heat production measured by indirect or direct calorimetry is reasonably accurate and precise, but measurement of NE requires measurement of the heat increment which cannot be measured directly. For nonruminants, after 12 to 24 hours of a meal, nutrient absorption from the gut is quite low, and therefore fasting heat production (FHP) can be measured by placing the animal in a calorimeter. After FHP is measured, the animal can be fed and heat production measured again. The difference between the two is the heat increment, and subtracting that value from ME and dividing by dry matter intake yields NE (Mcal/kg or lb). Because of the rumen, absorption of nutrients from the gut continues long after a meal (days), and therefore, FHP cannot be truly measured in ruminants. If FHP cannot be measured, then heat increment cannot be measured. For ruminants, heat increment is estimated by assuming a certain amount of heat is equal to maintenance or by regressing ME intake on heat production. Regardless of the method used to determine or estimate heat increment, accuracy and precision can be an issue. In addition to factors affecting ME, type of carbohydrate, concentrations of dietary fat and protein, and animal factors affect the efficiency of converting ME to NE. On average, as dietary concentrations of fiber and (or) protein increase, the efficiency of converting ME to NE decreases, and as dietary fat and starch increase, the efficiency of converting ME to NE increases. The reason protein has a low efficiency is because the excess amino acids have to undergo several reactions to be converted into other usable compounds, and each reaction produces heat (this is one of the reasons an Atkins-type diet can cause weight loss in humans). Dietary fat may participate in no additional chemical reactions and therefore produce no additional heat (extremely efficient). Measurement of NE includes all the errors associated with measuring GE, DE, and ME plus the errors associated with measuring heat increment. Therefore, NE is the least accurate and precise measure of diet energy. Even though NE may lack precision and accuracy, it has great theoretical advantages over other expressions of energy. The main advantage is that, unlike other expressions of energy, the use of NE allows different efficiency values to be used for different physiological activities (e.g., growth, maintenance, lactation).
ME versus NE Systems
Energy is lost by an animal as either chemical energy (feces, urine, methane) or heat. Both ME and NE account for all the chemical energy lost during the assimilation of nutrients, and both systems are commonly used in animal and human nutrition. The only difference between ME and NE is heat associated with inefficiency, therefore:
NE = ME * k where k = efficiency.
If efficiency (q) of utilization of all nutrients for all biological processes was the same, then ME would be just as accurate as NE (all we would be doing is multiplying by a constant). However, source of nutrients and the biological function supported by the nutrients affect efficiency. Reynolds et al. (1991) fed growing beef heifers diets with 75% alfalfa hay and 25% concentrate or 25% alfalfa hay and 75% concentrate (concentrate was mostly corn grain). Feed intake of the high-concentrate diet was limited so that both groups of heifers consumed 77 Mcal of ME/day (ME of the diets was measured), but heifers fed the high-concentrate diet gained significantly more weight than animals fed the high-forage diets. Based on ME, both diets should have performed equally, but because more of the ME on the high-forage diet was converted to heat, growth was not equal. This study (and many others) shows that source of ME (whether fat, protein, fiber, or starch) affects the efficiency of converting ME to NE, but currently available models generally only indirectly incorporate different efficiencies for different energy sources. Most animal nutrition models, however, do account for different efficiencies associated with various biological functions. We currently use mostly aggregated terms for energy requirements. For example, we do not determine the energy required for the heart to beat, for a muscle cell to synthesize protein, or for a mammary gland cell to synthesize lactose; rather, we have requirements for maintenance, growth, lactation, and pregnancy, and most current nutrition models use different ME to NE efficiencies for those aggregated functions. In general, the efficiency of converting ME to NE used for maintenance and lactation is similar (approximately 65%) and greater than the efficiency of converting ME to NE for growth (approximately 35%).
The Heat Increment
Heat increment can be a difficult concept, and it is very difficult to determine accurately. Animals, if they are alive, continually produce heat, and the heat increment may or may not be part of this heat. Heat increment cannot be measured directly; it is the difference obtained when heat production in a fasted (usually a 12- to 24-hour fast) animal is subtracted from heat production in a fed animal. Heat increment is therefore all the heat produced by the act of eating and digesting food and absorbing and transporting the nutrients from the gut. These activities are not the major component of heat increment, but they are important and account for some of the differences in efficiencies among different nutrients. For example, it takes more work (and therefore more heat is produced) for a cow to chew and digest a high-fiber diet than a high-concentrate diet. The major source of heat increment (and therefore the major source of variation in the efficiencies of converting ME to NE) is metabolism of the absorbed nutrients. Heat increment is not actually the heat produced when nutrients are metabolized; it is the difference in heat production when dietary nutrients are metabolized compared with heat produced when stored body nutrients are metabolized. When an animal is fasted (i.e., very few nutrients are being absorbed from the gut), metabolism does not stop (e.g., the heart must keep beating, and dead cells must be replaced). Since nutrients are not being absorbed from the gut, body stores of fat, glycogen, and amino acids must be used. When the animal is fed, absorbed nutrients are used instead of body stores. Heat increment represents the difference in efficiency between using absorbed nutrients and using stored nutrients for bodily functions. For example, the amount of heat produced when a cow used absorbed propionate to make lactose would be more than the heat produced if stored glucose (i.e., glycogen) was used to make lactose. The difference is heat increment.
Energy Requirements
Animals do not really have an energy requirement; rather, they have requirements for ATP and the substrates that produce ATP. Energy was something we could measure, and therefore energy systems were developed as proxies to the requirements for ATP-generating compounds. Undoubtedly, as our knowledge base, computing capacity, and analytical abilities increase, practical nutritional models will be developed that do not include energy but rather specific chemical substances. But essentially all currently used models include energy requirements for maintenance, growth, lactation, and pregnancy.
The NE requirements for growth, lactation, and pregnancy are simply the energy retained in the product (i.e., milk, tissue, or fetus). If a cow produces 100 lb of milk in a day and a sample of that milk produces 0.3 Mcal/lb of heat when it is completely combusted, then the NE requirement for lactation is 100 x 0.3 = 30 Mcal/day. Similarly, if a steer is growing at the rate of 4 lb/day and we know that 1 lb of the tissue it is accreting produces 1.2 Mcal of heat when completely combusted, the NE requirement for growth is 1.2 x 4 = 4.8 Mcal/day. The ME requirement for those processes in theory is NE divided by efficiency. Theoretical calculations of the energy efficiency of synthesizing milk fat, milk protein, and lactose from various substrates have been made and average 81, 89, and 77%, respectively. However, the NRC (2001) uses an efficiency of 64% for converting ME to NE in milk. The reason for this difference is that the theoretical calculations only account for entropy (i.e., heat produced because of inefficiency), but in a cow numerous other events (e.g., increased cardiac activity because of the need for increased blood flow to the mammary gland) must occur to support lactation, and these events are not 100% efficient and therefore produce heat.
Growth is a relatively inefficient process (approximately 35 to 50% of ME consumed above maintenance by a growing animal is retained in tissue, and 50 to 65% is lost as heat). Reasons for the low efficiency include the numerous reactions (heat is a by-product of every reaction) required to synthesize protein (starting at the production of mRNA and ending with a protein molecule), turnover of newly synthesized protein, conversion of dietary carbohydrate (most food animals are fed high-carbohydrate diets rather than high-fat diets) into stored fat, and additional physiological events are needed to support the newly synthesized tissues, all of which produce heat.
The efficiency of ME use for pregnancy is extremely difficult to determine. Estimates for laboratory animals and humans are 60 to 70%, whereas estimates for ruminants range from 10 to 20%. Part of the difference is caused by the methods used to obtain the estimates, and the true value probably lies somewhere between those extremes. The efficiency of converting ME to NE retained in a fetus should be less than efficiency of retaining energy as growth in free-living animals because the fetus also has a maintenance requirement, and energy used for maintenance is completely converted to heat. This heat will reduce efficiency. In addition, maternal heat production also increases as gestation progresses (for example, increased blood flow to support the fetus), and this heat reduces efficiency.
The basic functions necessary for life (i.e., maintenance) result in the formation of no chemical energy. All the chemical energy used for maintenance functions eventually is lost as heat because, by definition, maintenance means no net increase in energy retention in a body. Because all the maintenance requirement results in heat, FHP can be used to estimate the maintenance requirement. Calculated across all species, FHP = ~70 kcal/kg BW0.75 (the Brody-Kleiber law) which is used as an estimate of the NE requirement for maintenance. To allow adequate energy for some physical activity necessary for life (e.g., standing and walking to a feeding bunk), the NE requirement for maintenance is often FHP times 1.1 to 1.15 so that NE requirement for maintenance = ~80kcal/kg BW0.75. As discussed above, FHP cannot be measured in ruminants. In Table 2, heat production for the dry and lactating cow is 135 and 280 kcal/kg BW0.75. That heat production, however, includes maintenance requirement, heat increment, and some physical activity. Because FHP cannot be measured in ruminants, the NE requirement is estimated by adjusting heat production of a non-gestating, non-lactating animal by an estimated heat increment, or heat production is regressed on ME intake, and the intercept is an estimate of maintenance. Either way, maintenance requirement is the least accurate of all NE requirements for ruminants, and errors in excess of 10% have been suggested (Ellis et al., 2006).
Energy Balance
The first law of thermodynamics states that if we can accurately estimate NE intake and NE requirements, any difference between the two must be accounted for by changes in body energy. If NE intake is less than requirements, that energy must be provided by body stores (i.e., fat), and if NE intake is greater than requirements, that energy must be retained in the body (usually as fat). Change in body energy reserves (usually measured as changes in body condition and body weight) must be used to evaluate the estimated energy concentration of diets. If the computer says the average cow in a group is consuming 4 Mcal/day more NE than used for maintenance, milk, growth, and gestation, then the average cow in that group should be retaining 4 Mcal of energy per day (equivalent to about 1.7 lb of body mass). If cows in that group are not getting fatter, then you need to re-evaluate energy concentrations used in diet formulation. Errors are more likely to occur in estimating the NE value of feeds, but certain errors in estimating NE requirements can occur. Many cows are now housed in large pens and have to walk long distances to get milked (often three times per day). This requires energy, and your calculated maintenance requirement may not account for this expenditure. Some data suggest that for dairy cows, the NRC maintenance requirement is 10 to 15% too low and should be increased (Ellis et al., 2006). Increased physical activity could account for a large portion of that difference. In summary, energy is the nebulous nutrient. Measuring concentrations of energy in feed is extremely difficult, and measuring some energy requirements is problematic. A good nutritionist should not hesitate to make adjustments to either feed energy values or requirements based on apparent energy balance (i.e., change in body condition) and experience.
Author Information
W. P. Weiss
Department of Animal Sciences
Ohio Agricultural Research and Development Center
The Ohio State University, Wooster
Resources
Ellis, J. L., F. Qiao, and J. P. Cant. 2006. Evaluation of net energy expenditures of dairy cows according to body weight changes over a full lactation. J. Dairy Sci. 89:1546-1557.
Kleiber, M. 1975. The Fire of Life. An introduction to animal energetics. Robert E. Krieger Publishing Co., Huntington, NY.
National Research Council. 2001. Nutrient Requirements of Dairy Cattle. 7th rev. ed. Natl. Acad. Press, Washington, DC.
Reynolds, C. K., H. F. Tyrrell, and P. J. Reynolds. 1991. Effects of diet forage-to-concentrate ratio and intake on energy metabolism in growing beef heifers: whole body energy and nitrogen balance and visceral heat production. J. Nutr. 121:994-1003.
Tine, M. A., K. R. McLeod, R. A. Erdman, and R. L. Baldwin VI. 2001. Effects of brown midrib corn silage on the energy balance of dairy cattle. J. Dairy Sci. 84:885-895.
