Introduction
Early identification of nonpregnant dairy cows and heifers post breeding can improve reproductive efficiency and pregnancy rate by decreasing the interval between AI services and increasing AI service rate. Thus, new technologies to identify nonpregnant dairy cows and heifers early after artificial insemination (AI) may play a key role in management strategies to improve reproductive efficiency and profitability on commercial dairy farms. Transrectal palpation is the oldest and most widely used method for early pregnancy diagnosis in dairy cattle (Cowie, 1948). However, a newer technology may someday replace transrectal palpation as the method of choice for pregnancy diagnosis in the dairy industry. Before this can occur, however, two events must transpire. First, a technology must be developed that exceeds transrectal palpation in one or more of the characteristics of the ideal early pregnancy test. Thus, the first part of this chapter defines the attributes of the ideal pregnancy test and describes the direct and indirect methods for pregnancy diagnosis in dairy cattle that are currently available or under development that have the potential to replace transrectal palpation. Second and no less important, this new technology must be practically integrated into a systematic on-farm reproductive management strategy and empirically demonstrated to exceed the status quo of the industry (i.e., transrectal palpation) in reproductive performance. Thus, the latter part of this chapter presents research data and their implication for all currently available strategies for early pregnancy diagnosis from one attempt to integrate early identification of nonpregnancy into a systematic synchronized breeding program. Finally, a future direction for research and technology in the area of early pregnancy diagnosis in dairy cattle is presented and the overall conclusions of the ideas presented herein are drawn.
Please check this link first if you are interested in organic or specialty dairy production
Return to Estrus as a Diagnostic Indicator of Pregnancy Status
Return to estrus from 18 to 24 days after AI is often considered by dairy farmers the easiest and least costly method for determining nonpregnancy in dairy cattle early post breeding. This assumption, however, is being challenged by new research and long-recognized reproductive problems. First, estrous detection efficiency is estimated to be less than 50% on most dairy farms in the United States (Senger, 1994). This is likely a result of the short duration of estrus behavior reported for lactating cows (Dransfield et al., 1998) and because cows display estrus behavior poorly when housed on concrete flooring (Vailes and Britt, 1990), a common housing situation for dairy cattle in many regions of the US and other countries. Second, estrous cycle duration varies widely among lactating dairy cows from the standard 21-day interval and averaged around 24 days with a high degree of variability among animals for noninseminated lactating dairy cows (Sartori et al., 2004). This variability makes it difficult to target detection of return to estrus for groups of animals receiving AI on the same day. Finally, the high rate of embryonic mortality in dairy cows can increase the interval from insemination to return to estrus for cows that maintain a pregnancy then loose that pregnancy later during gestation. Although embryonic mortality is not the focus of this chapter, the rate of embryonic mortality during the period of gestation when dairy cattle are submitted for pregnancy examinations using methods described in this chapter is high and therefore is a key factor for understanding the implementation and implications of methods for early pregnancy diagnosis.
Embryonic Mortality in Dairy Cattle
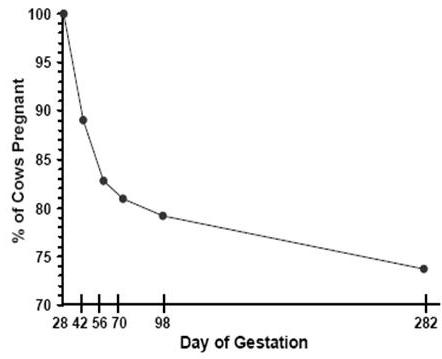
Embryonic mortality contributes to reproductive inefficiency because fertility assessed at any point during pregnancy is a function of both conception rate and embryonic mortality (Fricke, 2002). Embryonic mortality can be monitored using a variety of techniques including measurement of milk progesterone concentration or pregnancy specific proteins, transrectal ultrasonography, and transrectal palpation. Since the widespread implementation of transrectal ultrasonography for reproductive research in cattle (Griffin and Ginther, 1992), several studies have reported rates of embryonic mortality during early gestation under field conditions. Table 1 summarizes reported rates of embryonic mortality in lactating dairy cows from an initial pregnancy diagnosis conducted 27 to 30 days post breeding to a subsequent pregnancy reassessment 14 to 42 days later. Taken together, average pregnancy loss reported in these studies exceeded 15%. Vasconcelos et al. (1997) characterized embryonic mortality at various stages of gestation using transrectal ultrasonography and reported pregnancy losses of 11% from 28 to 42 d, 6% from 42 to 56 d, and 2% from 56 to 98 d post AI (Figure 1), suggesting that the rate of loss is higher early during gestation, then decreases as gestation proceeds.
Early pregnancy diagnosis can improve reproductive performance by decreasing the interval between successive AI services and coupling a nonpregnancy diagnosis with an aggressive strategy to rapidly rebreed these animals (Fricke, 2002). Conversely, it has long been accepted that pregnancy status should be determined in dairy cattle as soon as possible after insemination but without having the diagnosis confounded by subsequent embryonic mortality (Studer, 1969; Melrose, 1979). Embryonic mortality diminishes the benefit of early pregnancy diagnosis in two ways. First, because of the high rate of embryonic mortality that occurs around the time during gestation that most direct and indirect pregnancy tests are performed (Table 1), the magnitude of pregnancy loss detected is greater the earlier post breeding that a positive diagnosis is made. Thus, the earlier that pregnancy is diagnosed post breeding, the fewer nonpregnant cows are identified to which a management strategy can be implemented to rebreed them. Second and more important, cows diagnosed pregnant earlier post breeding have a greater risk for embryonic mortality compared to cows diagnosed later post breeding. If left unidentified, cows diagnosed pregnant early post breeding that subsequently loose that pregnancy reduce reproductive efficiency by extending the interval from calving to the conception that results in a full-term pregnancy.
To compensate for embryonic mortality, cows diagnosed pregnant early post breeding must undergo one or more subsequent pregnancy examinations to identify and rebreed cows that experience embryonic mortality. This applies to all methods for early pregnancy diagnosis including transrectal palpation conducted before the rate of embryonic mortality decreases. Thus, dairy mangers who have implemented early pregnancy diagnoses must consider the timing and frequency of subsequent pregnancy examinations to maintain the reproductive performance of the herd. Problems caused by embryonic mortality apply to all currently available methods for assessing pregnancy status early post breeding, and may relegate pregnancy testing before 30 to 40 days post breeding an untenable management strategy unless pregnancy diagnoses can be made continually on a daily basis or at each milking until the rate of embryonic mortality decreases (see Future Technologies section), or until the underlying causes of embryonic mortality are understood and mitigated.
Attributes of the Ideal Pregnancy Test
For successful integration into a reproductive management system, an ideal early pregnancy test for dairy cattle would be 1) sensitive (i.e., correctly identify pregnant animals) 2) specific (i.e., correctly identify nonpregnant animals), 3) inexpensive, 4) simple to conduct under field conditions, and 5) able to determine pregnancy status at the time the test is performed. Most direct and indirect methods for pregnancy diagnosis exhibit one or more of these attributes, but none currently available or under development exhibit all of them. A final attribute of an ideal test would be the ability to determine pregnancy status without the need to physically handle the animal to administer the test. Such a test may overcome the inherent limitations of current tests caused by embryonic mortality and may make pregnancy diagnosis before 30 to 40 days postpartum in dairy cattle an economically viable reproductive management strategy. Although all of the methods described in this chapter require animal handling to administer the test, future strategies and technologies for early pregnancy diagnosis may someday realize this goal.
Any direct or indirect method for pregnancy diagnosis must accurately distinguish between pregnant (i.e., test sensitivity) and nonpregnant (i.e., test specificity) animals. Inaccurate diagnosis of nonpregnancy (i.e., false negatives) consequently increases the rate of iatrogenic embryonic mortality when PGF2α or one of its analogues is administered to synchronize estrus or ovulation to reduce the interval to the next AI service. Because a management intervention can only be implemented for nonpregnant animals, it is critical that a pregnancy test accurately identifies nonpregnancy. Although inaccurate diagnosis of pregnancy (i.e., false positives) is undesirable, its consequences are less severe compared with inaccurate diagnosis of nonpregnancy. Nonetheless, a high rate of false positive results diminishes the usefulness and cost effectiveness of an early pregnancy test by incorrectly diagnosing animals as pregnant, thereby failing to present a management opportunity to return nonpregnant animals to AI service early post AI and potentially increasing the interval to the subsequent AI service.
Direct Methods for Pregnancy Diagnosis
By definition, direct methods for early pregnancy diagnosis of dairy cattle involve direct detection of the tissues and/or associated fluids of the conceptus either manually or via electronic instrumentation. Currently available direct methods for diagnosis of pregnancy include transrectal palpation and B-mode ultrasonography, and both methods are currently used by bovine practitioners to diagnose pregnancy in dairy cattle. Because they are direct methods, the test outcome can be subjective among practitioners. Technical expertise, operator proficiency, and the stage post breeding that the technique is performed can affect the specificity and sensitivity of the test. However, experienced practitioners can achieve acceptably high sensitivity and specificity with either method.
Transrectal Palpation
Transrectal palpation of the uterus for pregnancy diagnosis in cattle was first described in the 1800’s (Cowie, 1948) and is the oldest and most widely used method for early pregnancy diagnosis in dairy cattle today. Palpation technique can vary among practitioners. Transrectal palpation of the amniotic vesicle as an aid in determining pregnancy status in dairy cattle was described by Wisnicky and Cassida (1948), whereas slipping of the chorioallantoic membranes between the palpator’s thumb and forefinger beginning on about day 30 of gestation was described by Zemjanis (1970). Veterinary schools across the US and in other countries continue to train their students in the art of transrectal palpation for diagnosis of pregnancy in dairy cattle.
Because pregnancy in cattle can be terminated by manual rupture of the amnionic vesicle (Ball and Carroll, 1963), many studies have investigated the extent of iatrogenic embryonic mortality induced by transrectal palpation. Several studies have suggested that examining pregnant cows early in gestation by transrectal palpation increases the risk of iatrogenic embryonic mortality (Abbitt et al., 1978; Franco et al., 1987; Paisley et al., 1978; Valliancourt et al., 1979; White et al., 1989), whereas other studies have suggested that cows submitted for transrectal palpation earlier during gestation had a decreased risk for abortion or that palpation had no effect on subsequent embryonic losses (Studer, 1969; Thurmond and Picanso, 1993). Although controversy still exists regarding the extent of iatrogenic embryonic mortality induced by transrectal palpation, other factors have a greater influence on calving rates than pregnancy examination by transrectal palpation (Thompson et al., 1994). Furthermore, because the risk of embryonic mortality is high during the period of gestation when cows are diagnosed pregnant by transrectal palpation (Table 1), and because most cows within a herd are submitted for pregnancy examination, it is impossible for dairy producers and veterinarians to distinguish between iatrogenic losses occurring due to transrectal palpation and spontaneous losses that would normally have occurred in these cows.
Because of its widespread use and the number of bovine practitioners trained to perform the procedure, transrectal palpation will likely remain a mainstay for pregnancy diagnosis in dairy cattle until a newer direct or indirect method for pregnancy diagnosis is developed that exceeds the technique in one or more of the attributes of the ideal pregnancy test. Furthermore, because of its widespread use, high accuracy, and relatively low cost per animal, transrectal palpation is the industry standard that newer direct and indirect methods for pregnancy diagnosis in dairy cattle must displace as the method of choice for pregnancy diagnosis. Although the rate of embryonic mortality attributable directly to transrectal palpation is minimal, there is a need for controlled studies comparing embryonic losses in palpated and non-palpated cows at various stages of gestation. Furthermore, because transrectal palpation is routinely performed no earlier than 30 to 40 days post breeding, some but not all of the negative impact of embryonic mortality discussed previously can be overcome compared with methods for early pregnancy diagnosis performed from 6 to 26 days post breeding.
B-Mode Ultrasonography
Applications of and detailed methods for performing transrectal ultrasonography for reproductive research have been reviewed and described in detail (Ginther, 1998; Griffin and Ginther, 1992). Most veterinary students continue to be taught that ultrasound is a secondary technology for bovine reproductive work; however, the information-gathering capabilities of ultrasonic imaging far exceed those of transrectal palpation (Ginther, 1995). Although early pregnancy diagnosis is among the most practical application for reproductive management using transrectal ultrasonography, additional information gathered using the technology that may be useful for reproductive management include evaluation of ovarian structures, identification of cows carrying twin fetuses, and determination of fetal sex (Fricke, 2002). Day of first detection of identifiable characteristics of the bovine conceptus using transrectal ultrasonography was first reported by Curran et al. (1986; Table 2). A fetal heartbeat can be visualized at around 21 d of gestation under controlled experimental conditions and using a high-quality scanner and transducer (Table 2), and represents the definitive characteristic for positive confirmation of a viable pregnancy using transrectal ultrasonography. Although the rate of pregnancy loss is significant in studies using ultrasound to assess the rate of loss (Table 1), the technique itself has not yet been implicated as a direct cause of embryonic mortality in cattle (Ball and Logue, 1994; Baxter and Ward, 1997). Ultrasound is a less invasive technique for early pregnancy diagnosis than is transrectal palpation (Paisley et al., 1978; Vaillancourt et al., 1979) and may minimize the rare incidence of palpation-induced abortions.
Under most on-farm conditions, pregnancy diagnosis can be rapidly and accurately diagnosed using ultrasound as early as 26 d post AI (Filteau and DesCôteaux, 1998; Kastelic et al., 1991). When conducted between 21 and 25 d post breeding, sensitivity and specificity of pregnancy diagnosis using ultrasound was 44.8% and 82.3%, respectively, but increased to 97.7% and 87.7%, respectively, when conducted between 26 and 33 d post AI (Pieterse et al., 1990). Sensitivity and specificity of pregnancy diagnosis in lactating dairy cows based on ultrasonographic detection of uterine fluid as well as embryonic membranes from 28 to 35 days after AI was 96% and 97%, respectively (Nation et al., 2003). Direct observation of a fetus using ultrasonography was more accurate than assays for the presence of pregnancy specific proteins in plasma, but resulted in more false negative diagnoses (Szenci et al., 1998). Pregnancy diagnosis in dairy heifers based on the presence of intraluminal uterine fluid before Day 16, however, is unreliable because small amounts of fluid are present in non-inseminated heifers as early as 10 days after estrus (Kastelic et al., 1991). For lactating dairy cows, ultrasonographic detection of uterine fluid as well as embryonic membranes from 28 to 35 days after AI was an accurate estimation of the presence of an embryo at the time of observation (Nation et al., 2003). Although ultrasound conducted at ≥ 45 days post breeding did not increase accuracy of pregnancy diagnosis for an experienced palpator, it may improve diagnostic accuracy of a less experienced one (Galland et al., 1994).
As a pregnancy diagnosis method, transrectal ultrasonography is accurate and rapid, and the outcome of the test is known immediately at the time the test is conducted. Veterinary-grade ultrasound machines equipped with one rectal transducer are expensive and cost $8,000 to $16,000, and the cost of this technology may limit its practical implementation (Fricke, 2002). Although dairy producers can purchase an ultrasound scanner and conduct pregnancy examinations on their own cows, they generally lack the knowledge, training, and experience required to accurately perform pregnancy examinations (Fricke, 2002). Transrectal ultrasonography is slowly being incorporated into reproductive management schemes in dairies primarily by bovine practitioners who have adopted this technology. The extent to which transrectal ultrasonography will displace transrectal palpation as the primary direct method for pregnancy diagnosis in dairy cattle remains to be seen; however, if current trends continue, transrectal ultrasonography may displace transrectal palpation as the direct method of choice for pregnancy diagnosis among bovine practitioners. Because many experienced bovine practitioners can accurately diagnose pregnancy as early as 35 days post breeding using transrectal palpation, pregnancy examination using transrectal ultrasonography at 26 to 28 days post breeding only reduces the interval from insemination to pregnancy diagnosis by 7 to 9 days. The rate of embryonic mortality and the efficacy of strategies to rebreed cows at various stages post breeding also play a role in determining the advantages and disadvantages on the timing of pregnancy diagnosis and resynchronization (Fricke et al., 2003). Research is needed to clarify these issues and economically assess the decision to displace transrectal palpation with transrectal ultrasonography.
Indirect Methods for Pregnancy Diagnosis in Dairy Cattle
Indirect methods for early pregnancy diagnosis use qualitative or quantitative measures of reproductive hormones at specific stages after AI or detect conceptus specific substances in maternal body fluids as indirect indicators of the presence of a viable pregnancy. Research to develop commercial indirect methods for pregnancy diagnosis continues because these methods are non-invasive and the tests can be marketed to and performed by dairy farmers or herd employees. Currently available methods or methods under development for indirect diagnosis of pregnancy include measurement of endocrine hormones such as progesterone, and pregnancy specific proteins such as pregnancy-associated glycoproteins or the early pregnancy factor.
Endocrine Hormones
Progesterone
Progesterone produced by the corpus luteum is required for maintenance of pregnancy in cattle. Because normally cycling cows and heifers spontaneously ovulate at the end of each estrous cycle, post breeding serum progesterone profiles are similar for animals in which conception fails or succeeds until interferon-τ produced by the embryo disrupts the luteolytic PGF2α release from the uterine endometrium to maintain the corpus luteum and extend the period of elevated progesterone beyond 16 to 18 days post breeding (Thatcher, 2001). Interferon-τ exerts its luteolytic effect by inhibiting endometrial expression of oxytocin receptors through which oxytocin stimulates pulsatile PGF2α release (Wolf et al., 2003). Thus, although low progesterone concentrations of at 18 to 24 days post breeding can accurately predict nonpregnancy, high progesterone concentration during this period is not a specific indicator of pregnancy due to variation among cows in estrous cycle duration and embryonic mortality.
Progesterone concentrations in milk or serum can be quantified using a laboratory RIA or ELISA procedures. Commercially available qualitative milk progesterone assays have been developed and marketed to the dairy industry in the US for many years. The advantages of these tests are that they can be conducted using a milk sample, which is easier for farm workers to collect than a blood sample, and the ability to conduct the test on the farm rather than sending the sample to a regional laboratory, which requires a minimum of 2 to 3 days turn around time to return the test information to the farm. Commercial cow-side milk progesterone tests conducted between 18 and 24 days post breeding have reported a specificity of around 98% (Gowan et al., 1982; Pennington et al., 1985; Wimpy et al., 1985; Nebel et al., 1987) and are the earliest proven method for identifying nonpregnant animals post breeding. However, sensitivity in all of these studies was markedly lower than specificity, probably due to embryonic mortality. It is because of this confounding due to embryonic mortality that the outcome of milk progesterone testing is useful only as a method for detecting nonpregnant animals 18 to 24 days post breeding. Although subtle but significant differences in serum progesterone concentrations have been reported between pregnant and nonpregnant cows early post breeding (Mann and Lamming, 2001; Gumen et al., 2003), assessment of plasma progesterone 4 to 8 days after AI was not an accurate method for identifying nonpregnant cows (Montgomery et al., 1985).
Although, quantitative progesterone assays conducted at regional laboratories as well as qualitative cow-side test kits have been widely available to the dairy industry for many years, progesterone testing as an indirect method for early diagnosis of nonpregnancy is not a widespread practice in the dairy industry in the US. Despite widespread availability of milk progesterone testing through the National Milk Board in the United Kingdom in 1987, only 6% of dairy farms routinely used the service (Booth, 1987). Specific reasons for the limited implementation of this technology may include its less than perfect accuracy and cost of the tests (Hickey, 1990). Nevertheless, several companies continue to manufacture and market cow-side milk progesterone tests for use as an indirect nonpregnancy test for dairy cattle.
Estrone Sulfate
Estrone sulfate is a conjugated estrogen that has been used to diagnose pregnancy using milk samples in cattle (Heap and Hamon, 1979). Unlike progesterone concentration, which is not directly related to pregnancy, estrone sulfate is produced specifically by the conceptus and, therefore, its presence is a direct indicator of pregnancy. In cows, concentrations of estrone sulfate detectable in the whey fraction of milk are similar to those in maternal plasma and increase from about 60 days in gestation to the end of lactation with a plateau reached by around 150 days in gestation (Heap and Hamon, 1979). However, because concentrations of estrone sulfate in maternal circulation are not reliably detectable until around 80 days in gestation, and are not present in all pregnant cows until 100 days in gestation, estrone sulfate cannot compete with progesterone to assess pregnancy status early post breeding in cattle (Hamon et al., 1981).
Pregnancy Associated Proteins
Proteins produced and secreted specifically by the placenta early during pregnancy are obvious candidates for development of an early pregnancy test. However, the proteins produced by the placenta vary widely among eutherotic mammals. For example, only the higher primates produce a chorionic gonadotropin (CG) homologous to the human protein (hCG) required for luteal support early during pregnancy, whereas only ruminant ungulates are known to produce type I interferon as an antiluteolytic hormone (Xie et al., 1997). Because cattle do not produce a chorionic gonadotropin, the placental hormone that modern rapid pregnancy tests for women are based upon (Thomas et al., 1986), research has focused on discovery and characterization of pregnancy-specific proteins suitable for determining pregnancy status in cattle early post breeding. Promising candidates that have been researched to date include the pregnancy associated glycoproteins (PAG) and the early pregnancy factor (EPF).
Pregnancy Associated Glycoproteins (PAG)
Bovine pregnancy-associated glycoproteins (PAG) were discovered through attempts to develop indirect early pregnancy tests in dairy cattle (Xie et al., 1997). In 1982, two proteins, pregnancy-specific protein (PSP) A and B, were isolated from bovine fetal membrane extracts (Butler et al., 1982). Whereas PSPA was identified as α-fetoprotein, PSPB was found to be specific to placenta (Butler et al., 1982). Development of a specific RIA for PSPB (Sasser et al., 1986) allowed for quantification of PSPB in maternal serum as an indirect method for pregnancy diagnosis and embryonic mortality in dairy cattle (Humblot et al., 1988a, b).
Molecular cloning and sequencing studies have revealed that PAG represent a large family of proteins expressed by placenta, with 21 PAG family members identified in cattle as of 2000 (Zoli et al., 1991; Green et al., 2000). Green et al. (2000) characterized temporal expression of PAG 1 through PAG 11 (numbered by their order of discovery) in bovine placenta throughout gestation using an RNase protection assay and found that patterns of expression differed among the various PAG (Figure 3). Both PSPB and PAG-1 have identical N-terminal sequences and are presumably identical or closely related proteins (Green et al., 2000). Although their function during pregnancy or parturition is unclear, Xie et al. (1991) demonstrated that PAG and its ovine homolog are inactive members of the aspartic proteinase family. If enzymatically active, PAG could be important in structural remodeling of the placenta during pregnancy or in placental detachment after parturition (Zoli et al., 1992b).
Formation of the blastocyst during early embryonic development separates cells of the morula into those that will develop into the embryo proper or trophoblast cells that will develop to form the placenta. Bovine PAG have been immunologically localized to trophoblast binucleate cells present in fetal cotyledonary villi and to a lesser extent to caruncular epithelium, and are stored before their release in intracellular granules (Zoli et al., 1992b). Trophoblast binucleate cells of ruminant ungulates can be first identified morphologically around the time of definitive cellular attachment of the trophoblast to the uterine epithelium (Wooding, 1992). Binucleate cells constitute around 20% of the trophoectoderm layer and are continually replaced as migration to the uterine epithelium proceeds (Wooding, 1992). Migration of binucleate cells from the trophectoderm to the uterine epithelium allows for exocytisis of granules containing PAG into the maternal circulation (Wooding, 1992).
Because cellular products of binucleate cells are released into maternal circulation, the ideal antigen for an indirect early pregnancy test in dairy cattle would be a PAG expressed in binucleate cells around the time of implantation (Green et al., 2000). Most studies have reported that in pregnant cows, mean PAG concentrations increase from 15 to 35 days in gestation, however, variation in serum PAG levels among animals precludes PAG as a reliable indicator of pregnancy until about 26 to 30 day in gestation (Humblot, 2001; Zoli et al., 1992a). Zoli et al. (1992a) developed a specific RIA to characterize PAG concentrations during pregnancy and after calving in dairy and beef cows. Serum PAG concentrations were 0.38 ± 0.13 ng/ml on Day 22 and rose continuously as pregnancy advanced until Day 220 of gestation (Figure 4). After Day 240, PAG concentrations were > 158.90 ± 60.20 ng/ml and rapidly increased to 1,551.90 ± 589.70 ng/ml by Day 270 with a dramatic increase to 2,462.40 ± 1,017.90 ng/ml at peak concentration around 5 days prepartum. After parturition, PAG concentrations decreased steadily to 499.60 ± 267.20 ng/ml at Day 14, 131.70 ± 77.90 ng/ml at Day 30 and 10.10 ± 7.80 ng/ml at Day 60, with undetectable levels achieved only by Day 100 ± 20 postpartum (Zoli et al., 1992a). Although pregnancy can be determined beginning 29 to 30 days post breeding, the presence of PAG in maternal serum for nearly 100 days postpartum constitutes a problem for diagnosis of pregnancy during the subsequent lactation if rebreeding occurs at less than 80 days postpartum (Zoli et al., 1992a). This does not preclude use of PAG tests for dairy cattle inseminated immediately at the end of a 60 day voluntary waiting period and submitted for a PAG-based pregnancy test 30 days post breeding, but dairy cows are submitted for first service earlier than 60 days postpartum in many dairy herds.
The sensitivity and specificity of PSPB based on RIA of serum samples was 92.0% and 82.6% to 91.9% beginning 29 to 30 days after insemination (Szenci et al., 1998). By contrast, the sensitivity and specificity of pregnancy testing using PAG 1 based on radioimmunoassay of serum samples was 95.2% to 100.0% and 56.7% to 79.0% beginning 29 to 30 days after AI (Szenci et al., 1998). Measurement of PAG in serum 35 days after estrus from Holstein heifers receiving embryo transfer resulted in an overall accuracy of 94.7% (Zoli et al., 1992a; Table 3).
Although PAG are presently one of the more promising markers for early detection of pregnancy in cattle, PAG-based pregnancy tests are limited by a number of factors. First, PAG is not detectable in milk or urine, and therefore requires that a blood sample be collected, a procedure that is difficult on some farms. Second, because PAG reaches high concentrations in maternal circulation during the periparturient period and has a long serum half-life, cows inseminated too early postpartum are not eligible for testing. Finally, because no qualitative cow-side PAG test has yet been developed, a strategy using regional laboratories for quantification of PAG using ELISA or RIA will be required for commercialization. Although this allows for quantification of serum PAG under controlled laboratory conditions, this strategy presents two difficulties. First, inaccuracies in labeling and tracking of animal identification and blood samples throughout the process from collection of the blood sample to the return of the pregnancy diagnosis information to the farm requires stringent quality control efforts by both on farm and laboratory personnel. Second and more important, there is a minimum turn around time of 2 to 3 days from blood sample collection to return of the diagnostic information to the farm. Depending on the rebreeding strategy in place on the farm, an extra animal handling period may be required to collect the blood sample for the test. This may be a challenge for larger dairies using systematic synchronization and resynchronization systems for reproductive management.
Early Pregnancy Factor
Early pregnancy factor (EPF) was first identified in pregnant mice (Morton et al., 1987) and later in sheep and cattle (Nancarrow et al., 1981) by using the rosette inhibition bioassay. With this assay, EPF was detected in the serum of all mammals tested within 24 to 48 h of fertilization, and disappeared within 24 to 48 after death or removal of the embryo (Morton et al., 1987). Because the developing embryo bears antigens foreign to the mother, immune rejection of the early embryo may occur. As an immunosuppressive factor present as early as 6 to 48 hours after mating, EPF may function to suppress the maternal immune response, thereby allowing for pregnancy to proceed (Shaw and Morton, 1980). Molecular cloning of the EPF gene has shown that EPF is a homolog of chaperonin 10 and is a member of a family of heat shock proteins (Summers et al., 1996; Morton, 1998). Although EPF was first described as a pregnancy-associated substance, recent studies suggest that EPF plays a broader role as a regulator of cell proliferation for both normal and neoplastic cells in vivo and in vitro (Morton et al., 1992; Cavanagh, 1996). Despite its wider role as a regulator of cellular proliferation, EPF is necessary for successful establishment of pregnancy. Passive immunization of mice with anti-EPF antibodies retarded embryonic development in the early pre-implantation stage and resulted in failure of implantation at the peri-implantation stage of development (Athanasas-Plastsis et al., 2000).
In cattle, significant differences in rosette inhibition titer were observed between pregnant and nonpregnant cows on Day 13-16 and 25 post breeding (Sakonju et al., 1993) suggesting that measurement of EPF activity may be useful as an indirect method for early pregnancy diagnosis. Antibodies generated against a glycoprotein immunosuppressive EPF isolated from sera of pregnant cows were used to detect EPF (Threlfall, 1994), and a cow-side test for EPF in cattle has been developed and marketed in the US. The commercially available Early Conception Factor (ECF) test (Concepto Diagnostics, Knoxville, TN) reportedly detects a pregnancy-associated glycoprotein within 48 h of conception. Two studies have compared results from the ECF test conducted between Days 3 to 7 and Days 11 to 15 post breeding to pregnancy diagnosis using transrectal palpation and ultrasound ranging from 25 to 60 days post breeding (Adams and Jardon, 1999; Des Côteaux et al., 2000). Both studies reported a poor accuracy for this commercial test at the time the studies were conducted. One concern with these assessments is that animals with viable embryos during early pregnancy that subsequently undergo embryonic mortality before pregnancy diagnosis using transrectal palpation or transrectal ultrasonography increase the rate of false positive results and bias the assessment. The fertilization rate after AI in beef cows is 90%, whereas embryonic survival rate is 93% by Day 8 and only 56% by Day 12 post AI (Diskin and Sreenan, 1980). Similarly, only 48% of embryos recovered from dairy cows on Day 7 after AI were classified as normal (Weibold, 1988). Thus, substantial pregnancy loss likely occurred before the establishment of pregnancy status using rectal palpation or transrectal ultrasonography in these studies.
To preclude the confounding effect of embryo loss, Cordoba et al. (2001) reported a study in which noninseminated Holstein cows and heifers were evaluated as an unequivocal source of nonpregnant animals, and Holstein cows and heifers inseminated at estrus and in which at least one embryo of transferable quality was recovered at a nonsurgical flush 6 d after artificial insemination were evaluated as an unequivocal source of pregnant animals. Blood samples were collected from all animals 6 d after estrus, which was immediately before embryo collection in pregnant animals. Day 6 after estrus was chosen as the day for the pregnancy test because, under field conditions, a nonpregnancy diagnosis could be coupled with administration of a luteolytic dose of PGF2α to hasten return to estrus. Each serum sample was evaluated using two ECF test cassettes, and the result of each test cassette was interpreted by two independent readers. Test sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 86%, 4%, 49%, 23%, and 46%, respectively (Table 4). Although the observed agreement between readers (91% for Test 1; 89% for Test 2) and between tests for the same serum sample (94% for Reader 1; 91% for Reader 2) was high, the overall rates of false positive and false negative ECF test results were 96% and 14%, respectively (Table 4). The authors concluded that the ECF test is an unreliable method for determining pregnancy status of dairy cattle on Day 6 after estrus due to the poor overall accuracy of the test. Clearly, the ECF test cannot be recommended in its tested form. It remains to be seen whether EPF can be developed into a commercially reliable early pregnancy test for cattle.
On Farm Implementation of Early Nonpregnancy Diagnosis
Synergies between new reproductive management technologies hold the key to maximizing reproductive efficiency on dairy farms. However, reproductive management protocols that allow for synchronization of estrus or ovulation and subsequent identification and resynchronization of nonpregnant cows must be practical to implement within the day to day operation of a dairy farm or the protocol will fail due to lack of compliance (Fricke et al., 2003). This is especially true for larger farms that must schedule and administer artificial inseminations, hormone injections, and pregnancy tests for a large number of animals on a daily or weekly basis. Identification of nonpregnant cows early post breeding can only improve reproductive efficiency when coupled with a management strategy to rapidly submit nonpregnant cows for a subsequent AI service. Thus, any direct or indirect method for early pregnancy diagnosis must be integrated as a component of the overall reproductive management strategy in place on the farm. The various component technologies of the reproductive management system will in turn determine the timing of the events as they occur on a daily or weekly basis. As stated previously, it has long been accepted that pregnancy status should be determined in dairy cattle as soon as possible after insemination but without having the diagnosis confounded by subsequent embryonic mortality (Studer, 1969; Melrose, 1979). New research on the practical implementation of early pregnancy diagnosis using transrectal ultrasonography into a systematic synchronization and resynchronization system has confirmed this notion and illustrated the pitfalls and limitations of early pregnancy diagnosis (Fricke et al., 2003).
Field Trial: Integrating Systematic Synchronization with Transrectal Ultrasonography
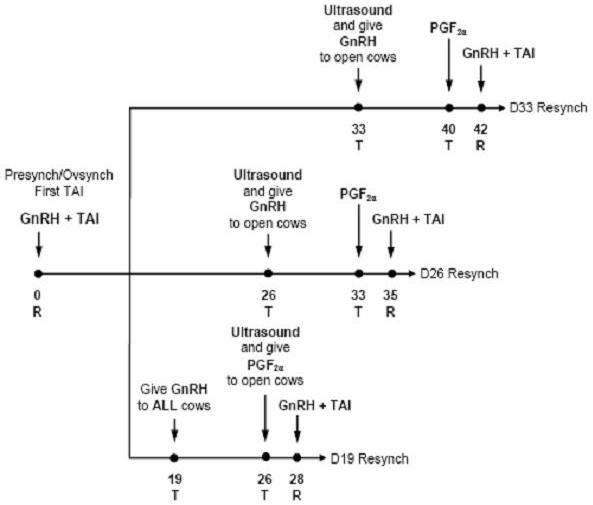
Two new and currently available technologies for reproductive management include hormonal protocols such as Ovsynch (Pursley et al., 1995, 1997) and Presynch/Ovsynch (Moreira et al., 2001; Navanukraw et al., 2004) that synchronize ovulation and allow for TAI, and use of transrectal ultrasonography for early identification of nonpregnant cows (Fricke, 2002). A field trial was conducted to compare three intervals from first TAI to resynchronization of ovulation on a dairy incorporating transrectal ultrasonography as a direct method for early pregnancy diagnosis (Fricke et al., 2003). The objective was to compare conception rate to first TAI service after a modified Presynch protocol with conception rates after resynchronization of ovulation using Ovsynch at three intervals post TAI (Resynch) coupled with pregnancy diagnosis using transrectal ultrasonography (Figure 2). Lactating dairy cows on a commercial dairy farm were enrolled into this study on a weekly basis. All cows received a modified Presynch protocol to receive first postpartum TAI as follows: 25 mg PGF2α (d 32 ± 3; d 46 ± 3); 50 μg GnRH (d 60 ± 3); 25 mg PGF2α (d 67 ± 3) and 50 μg GnRH (d 69 ± 3) postpartum (Navanukraw et al., 2004). All cows received TAI immediately after the second GnRH injection of the Presynch protocol (d 0) as per a Cosynch TAI schedule. At first TAI, cows were randomly assigned to each of three treatment groups for resynchronization of ovulation (Resynch) using Ovsynch [50 μg GnRH (d -9); 25 mg PGF2α (d -2) and 50 μg GnRH + TAI (d -0)] to induce a second TAI for cows failing to conceive to first TAI service. All cows (n=235) in the first group (Day 19) received a GnRH injection on d 19 post TAI and continued the Ovsynch protocol if diagnosed nonpregnant using transrectal ultrasound on d 26 post TAI. Cows (n=240) in the second (Day 26) and cows (n=236) in the third (Day 33) groups initiated the Ovsynch protocol if diagnosed nonpregnant using transrectal ultrasound on d 26 post-TAI or d 33 post-TAI, respectively.
Submission of cows for first postpartum TAI service was scheduled so that the first four injections of the Presynch plus Ovsynch protocol occurred on Tuesdays followed by the second GnRH injection and TAI occurring on Thursdays (Table 5; Figure 2). Initiation times for Resynch for each of the three treatment groups in this study were chosen to occur on Tuesdays so that injection schedules would remain consistent for all cows assigned to weekly breeding groups at any given time. To adhere to the Tuesday/Thursday schedule, all pregnancy examinations were conducted on Tuesdays. To fit the reproductive management system, the first pregnancy examination using transrectal ultrasound was conducted 26 d after TAI for the D19 and D26 cows and 33 d after TAI for the D33 cows (Figure 2). Thus, the reproductive management systems assessed in this trial allow for administration of all hormone injections, Ovsynch and Resynch TAI services, and pregnancy examinations to be restricted regularly to either Tuesdays or Thursdays (Table 5).
Implicit to the experimental design, first assessment of pregnancy status was not conducted at the same interval after the Ovsynch TAI among the three treatment groups (Figure 2). Pregnancy status after the Ovsynch TAI was first assessed 26 d after TAI for cows in the D19 and D26 groups, whereas pregnancy status was assessed 33 d post Ovsynch TAI for cows in the D33 group. Overall PR/AI to Ovsynch was 40% and was greater for D19 and D26 cows than for D33 cows (Table 6). This difference is likely due to a greater period in which embryonic mortality can occur in the D33 cows due to the increased interval from TAI to pregnancy diagnosis (26 vs. 33 d). When pregnancy status was reassessed for all treatment groups at 68 d after Ovsynch TAI, overall PR/AI to Ovsynch was 31% and did not differ among treatments (Table 6). Thus, differences in PR/AI at the first pregnancy exam and pregnancy losses between the first and second pregnancy exams among treatment groups likely represent an artifact of time of assessment of pregnancy status after TAI inherent to the experimental design rather than to treatment differences. Overall PR/AI to Resynch was 32% and was greater for D26 and D33 cows than for D19 cows (Table 7).
The Challenges for Early Pregnancy Diagnosis
Data from Tables 6 and 7 illustrate the limitations of integrating early pregnancy diagnosis into a reproductive management program. First, the system with the most aggressive early nonpregnancy diagnosis and resynchronzation schedule (i.e., the D19 treatment) was not a viable management strategy based on the poor fertility after the Resynch TAI (Table 7) probably due to follicular and luteal dynamics at the stage post breeding that the synchronization protocol was initiated. Furthermore, these results suggest the counterintuitive notion that delaying pregnancy diagnosis from 26 to 33 days post TAI may improve reproductive efficiency when using a hormonal protocol for timed AI to program nonpregnant cows for rebreeding due to the high rate of embryonic mortality occurring in cows diagnosed pregnant at 26 vs. 33 days post TAI (Table 6).
Future Technologies
Although several direct and indirect methods for early pregnancy diagnosis are available for dairy cattle, none of these methods fulfill all of the attributes of the ideal early pregnancy test. Furthermore, refinement of current technologies and new technologies under development will not likely fulfill the characteristics of the ideal early pregnancy test due to inherent limitations regarding the nature of the tests and the strategies they employ. If an ideal early pregnancy test is to be developed, a novel approach must be taken.
A novel approach to the problem of early pregnancy diagnosis in dairy cattle would be to monitor a pregnancy specific substance or hormone secreted in milk in sufficient quantities to be detected by an in-line milk sensing device during normal milking periods on a dairy. Obviously, this pregnancy specific substance must first be discovered or a known marker must be used, and the in-line milk sampling technology developed to accurately detect and monitor this substance. If sensitive and specific, such a system would have a minimal marginal cost per test once the initial capital outlay was made to install the equipment on the dairy. By using such a system, a pregnancy diagnosis would be conducted during each milking period for all lactating cows on a dairy so that nonpregnancy, pregnancy, and pregnancy loss could be continually monitored and tracked on a daily basis. Integration of this information into a computerized dairy management software system would allow dairy managers to review the pregnancy status of individual cows in the herd on a daily or weekly basis so that reproductive management strategies could be implemented to establish, maintain, or attempt to reinitiate a pregnancy. Finally, such a system would achieve the heretofore unrealized characteristic of conducting the pregnancy test without specifically handling the animal to administer the test. Limitations imposed by embryonic mortality during early gestation will not be overcome until such a system is developed. It will indeed be exiting to witness what the future holds with regard to methods for early pregnancy diagnosis in dairy cattle.
Conclusion
Although coupling a nonpregnancy diagnosis with a management decision to quickly reinitiate AI service may improve reproductive efficiency by decreasing the interval between AI services, early embryonic mortality and the effectiveness of hormonal ovulation and estrus control protocols initiated at certain physiologic stages post breeding may limit the effectiveness of many direct and indirect methods for early pregnancy diagnosis currently under development, especially when compared to transrectal palpation. These limitations make the benefits of many currently available methods for early pregnancy diagnosis questionable and require that all animals diagnosed pregnant early after insemination be scheduled for rechecks at later times during gestation to identify animals experiencing embryonic mortality. Although much research and development efforts are being made toward development of an indirect pregnancy test for cattle, it remains to be seen whether a new direct or indirect test will replace transrectal palpation as the primary method used for pregnancy diagnosis in dairy cattle. Future technologies for pregnancy diagnosis in dairy cattle may someday overcome these limitations thereby improving reproductive performance.
Literature Cited
- Abbitt BL Ball G, Kitto P, Sitzman CG, Wilgenburg B, Raim LW, Seidel GE Jr. Effect of three methods of palpation for pregnancy diagnosis per rectum on embryonic and fetal attrition in cows. J Am Vet Med Assoc 1978; 173:973-977.
- Adams CS, Jardon PW. Evaluation of the early conception factor tests in cows 3-7 days post-571 breeding. Proc Am Assoc Bov Pract 1999; 32:240-241.
- Athanasas-Platsis S, Corcoran CM, Kaye PL, Cavanagh AC, Morton H. Early pregnancy factor is required at two important stages of embryonic development in the mouse. Am J Reprod Immunol 2000; 43:223-233.
- Ball L, Carroll EJ. Induction of fetal death in cattle by manual rupture of the amniotic vesicle. J Am Vet Med Assoc 1963; 142:373-374.
- Ball PJH, Logue DDN. Ultrasound diagnosis of pregnancy in cattle. Vet Rec 1994; 134:532.
- Baxter SJ, Ward WR. Incidence of fetal loss in dairy cattle after pregnancy diagnosis using an ultrasound scanner. Vet Rec 1997; 140:287-288.
- Booth JM. Pregnancy testing today. Br Vet J 1987; 143:385-386.
- Butler JE, Hamilton WC, Sasser RG, Ruder CA, Haas GM, Williams RJ. Detection and partial characterization of two bovine pregnancy-specific proteins. Biol Reprod 1982; 26:925-933.
- Cartmill JA, El-Zarkouny SZ, Hensley BA, Lamb GC, Stevenson JS. Stage of cycle, incidence, and timing of ovulation, and pregnancy rates in dairy cattle after three timed breeding protocols. J Dairy Sci 2001a; 84:1051-1059.
- Cartmill JA, El-Zarkouny SZ, Hensley BA, Rozell TG, Smith JF, Stevenson JS. An alternative AI breeding protocol for dairy cows exposed to elevated ambient temperatures before or after calving or both. J Dairy Sci 2001b; 84:799-806.
- Cavanagh A. Identification of early pregnancy factor as chaperonin 10: implications for understanding its role. Rev Reprod 1996; 1:28-32.
- Chebel RC, Santos JEP, Juchem SO, Cerri RLA, Galvao KN, Thatcher WW. Effect of resynchronization with GnRH on day 21 after artificial insemination on conception rate and pregnancy loss in lactating dairy cows. Theriogenology 2003; 60:1389-1399.
- Cordoba MC, Sartori R, Fricke PM. Assessment of a commercially available Early Conception Factor (ECF) test for determining pregnancy status of dairy cattle. J Dairy Sci 2001; 84:1884-1889.
- Cowie TA. Pregnancy diagnosis tests: A review. Commonwealth Agricultual Bureaux Joint Publication No. 13, Great Britain, 1948 pp 11-17.
- Curran S, Pierson RA, Ginther OJ. Ultrasonographic appearance of the bovine conceptus from days 20 through 60. J Am Vet Med Assoc 1986; 189:1295-1302.
- Des Côteaux L, Carrière PD, Bigras-Poulin M. Evaluation of the Early Conception Factor (ECF) dipstick test in dairy cows between days 11 and 15 post-breeding. Bov Pract 2000; 34:87-91.
- Diskin MG, Sreenan JM. Fertilization and embryonic mortality rates in beef heifers after artificial insemination. J Reprod Fertil 1980; 59:463-468.
- Dransfield MBG, Nebel RL, Pearson RE, Warnick LD. Timing of artificial insemination for dairy cows identified in estrus by a radiotelemetric estrus detection system. J Dairy Sci 1998; 81:1874-1882.
- Filteau V, DesCôteaux L. Predictive values of early pregnancy diagnosis by ultrasonography in dairy cattle. Proc AABP Annu Mtg, Spokane, WA, 1998; 31:170-171.
- Franco OJ, Drost M, Thatcher MJ, Shille VM, Thatcher WW. Fetal survival in the cow after pregnancy diagnosis by palpation per rectum. Theriogenology 1987; 27:631-644.
- Fricke PM. Scanning the future – Ultrasonography as a reproductive management tool for dairy cattle. J Dairy Sci 2002; 85:1918-1926.
- Fricke PM, Caraviello DZ, Weigel KA, Welle ML. Fertility of dairy cows after resynchronization of ovulation at three intervals after first timed insemination. J Dairy Sci 2003; 86:3941-3950.
- Fricke PM, Guenther JN, Wiltbank MC. Efficacy of decreasing the dose of GnRH used in a protocol for synchronization of ovulation and timed AI in lactating dairy cows. Theriogenology 1998; 50:1275-1284.
- Galland JC, Offenbach LA, Spire MF. Measuring the time needed to confirm fetal age in beef heifers using ultrasonographic examination. Vet Med 1994; 89:795-804.
- Ginther OJ. Ultrasonic imaging and animal reproduction: Fundamentals. Book 1. Equiservices Publishing, Cross Plains, WI, 1995.
- Ginther OJ. Ultrasonic imaging and animal reproduction: Cattle. Book 3. Equiservices Publishing, Cross Plains, WI, 1998.
- Gowan EW, Etches RJ, Bryden C, King GJ. Factors affecting accuracy of pregnancy diagnosis in cattle. J Dairy Sci 1982; 65:1294-1302.
- Green JA, Xie S, Quan X, Bao B, Gan X, Mathialagan N, Beckers JF, Roberts RM. Pregnancy-associated bovine and ovine glycoproteins exhibit spatially and temporally distinct expression patterns during pregnancy. Biol Reprod 2000; 62:1624-163131.
- Griffin PG, Ginther OJ. Research applications of ultrasonic imaging in reproductive biology. J Anim Sci 1992; 70:953-972.
- Gümen A, Guenther JN, Wiltbank MC. Follicular size and response to Ovsynch versus detection of estrus in anovular and ovular lactating dairy cows. J Dairy Sci 2003; 86:3184-3194.
- Hamon M, Fleet IR, Holdsworth RJ, Heap RB. The time of detection of oestrone sulphate in milk and the diagnosis of pregnancy in cows. Br Vet J 1981; 137:71-77.
- Heap RB, Hamon M. Oestrone sulphate in milk as an indicator of a viable conceptus in cows. Br Vet J 1979; 135:355-363.
- Hickey GJ. Pregnancy diagnosis in dairy cattle: present status and future prospects. Cornell Vet 1990; 80:299-302.
- Humblot P. Use of pregnancy specific proteins and progesterone assays to monitor pregnancy and determine the timing, frequencies and sources of embryonic mortality in ruminants. Theriogenology 2001; 56:1417-1433.
- Humblot P, Camous S, Martal J, Charlery J, Jeanguyot N, Thibier M, Sasser RG. Diagnosis of pregnancy by radioimmunoassay of a pregnancy-specific protein in the plasma of dairy cows. Theriogenology 1988a; 30:257-268.
- Humblot P, Camous S, Martal J, Charlery J, Jeanguyot N, Thibier M, Sasser RG. Pregnancy-specific protein B, progesterone concentrations and embryonic mortality during early pregnancy in dairy cows. J Reprod Fertil 1988b; 83:215-223.
- Kastelic JP, Bergfelt DR, Ginther OJ. Ultrasonic detection of the conceptus and characterization of intrauterine fluid on days 10 to 22 in heifers. Theriogenology 1991; 35:569-581.
- Mann GE, Lamming GE. Relationship between maternal endocrine environment, early embryo development and inhibition of the luteolytic mechanism in cows. Reproduction 2001; 121:175-180.
- Melrose DR. The need for, and possible methods of application of, hormone assay techniques for improving reproductive efficiency. Br Vet J 1979; 135:453-459.
- Montgomery ME, Leslie KE, Martin SW. The sensitivity and specificity of postbreeding plasma progesterone levels as a pregnancy test for dairy cows. Can J Comp Med 1985; 49:346-349.
- Moreira F, Orlandi C, Risco CA, Mattos R, Lopes F, Thatcher WW. Effects of pre- synchronization and bovine somatotropin on pregnancy rates to a timed artificial insemination protocol in lactating dairy cows. J Dairy Sci 2001; 84:1646-1659.
- Morton H. Early pregnancy factor: an extracellular chaperonin 10 homologue. Immunol Cell Biol 1998; 76:483-96.
- Morton H, Cavanagh AC, Athanasas-Platsis S, Quinn KA, Rolfe BE. Early pregnancy factor has immunosuppressive and growth factor properties. Reprod Fertil Dev 1992; 4:411-422.
- Morton H, Rolfe BE, Cavanagh AC. Ovum factor and early pregnancy factor. Curr Topics Dev Biol 1987; 23:73-92.
- Navanukraw C, Reynolds LP, Kirsch JD, Grazul-Bilska AT, Redmer DA, Fricke PM. A modified presynchronization protocol improves fertility to timed artificial insemination in lactating dairy cows. J Dairy Sci 2004; 87:1551-1557.
- Nancarrow CD, Wallace ALC, Grewal AS. The early pregnancy factor of sheep and cattle. J Reprod Fertil (Suppl) 1981; 30:191-199.
- Nation DP, Malmo J, Davis GM, Macmillan KL. Accuracy of bovine pregnancy detection using transrectal ultrasonography at 28 to 35 days after insemination. Aust Vet J 2003; 81:63-65.
- Nebel RL, Whittier WD, Cassell BG, Britt JH. Comparison of on-farm and laboratory milk progesterone assays for identifying errors in detection of estrus and diagnosis of pregnancy. J Dairy Sci 1987; 70:1471-1476.
- Paisley LG, Mickelsen WD, Frost OL. A survey of the incidence of prenatal mortality in cattle following pregnancy diagnosis by rectal palpation. Theriogenology 1978; 9:481-489.
- Pennington JA, Schultz LH, Hoffman WF. Comparison of pregnancy diagnosis by milk progesterone on day 21 and day 24 postbreeding: field study in dairy cattle. J Dairy Sci 1985; 68:2740-2745.
- Pieterse MC, Szenci O, Willemse AH, Bajcsy CSA, Dieleman SJ, Taverne MAM. Early pregnancy diagnosis in cattle by means of linear-array real-time ultrasound scanning of the uterus and a qualitative and quantitative milk progesterone test. Theriogenology 1990; 33:697-707.
- Pursley JR. Kosorok MR, Wiltbank MC. Reproductive management of lactating dairy cows using synchronization of ovulation. J Dairy Sci 1997; 80:301-306.
- Pursley JR, Mee MO, Wiltbank MC. Synchronization of ovulation in dairy cows using PGF2α and GnRH. Theriogenology 1995; 44:915-923.
- Sakonju S, Enomoto S, Kaminura S, Amana K. Monitoring bovine embryo viability with 698 early pregnancy factor. J Vet Med Sci 1993; 55:271-274.
- Santos JEP, Bartolome JA, Cerri RLA, Juchem SO, Thatcher WW, Hernandez O, Trigg T. Effect of a deslorelin implant in a timed artificial insemination protocol on follicle development, luteal function and reproductive performance of lactating dairy cows. Theriogenology 2004b; 61:421-435.
- Santos JEP, Juchem SO, Cerri RLA, Galvão KN, Chebel RC, Thatcher WW, Dei C, Bilby C. Effect of bST and reproductive management on reproductive performance of Holstein dairy cows. J Dairy Sci 2004a; 87:868-881.
- Santos JEP, Thatcher WW, Pool L, Overton MW. Effect of human chorionic gonadotropin on luteal function and reproductive performance of high-producing lactating Holstein dairy cows. J Anim Sci 2001; 79:2881-2894.
- Sartori R, Haughian JM, Shaver RD, Rosa GJM, Wiltbank MC. Comparison of ovarian function during the estrous cycle of Holstein heifers and lactating cows. J Dairy Sci 2004; In press.
- Sasser RG, Ruder CA, Ivani KA, Butler JE, Hamilton WC. Detection of pregnancy by radioimmunoassay of a novel pregnancy-specific protein in serum of cows and a profile of serum concentration during gestation. Biol Reprod 1986; 35:936-942.
- Senger PL. The estrus detection problem: new concepts, technologies, and possibilities. J Dairy Sci 1994; 77:2745-2753.
- Shaw FD, Morton H. The immunological approach to pregnancy diagnosis: a review. Vet Rec 1980; 106:268-270.
- Silke V, Diskin MG, Kenny DA, Boland MP, Dillon P, Mee JF, Sreenan JM. Extent, pattern and factors associated with late embryonic loss in dairy cows. Anim Reprod Sci 2002; 71:1-12.
- Studer E. Early pregnancy diagnosis and fetal death. Vet Med Small Anim Clin 1969; 64:613-617.
- Summers KM, Murphy RM, Webb GC, Peters GB, Morton H, Cassady AI, Cavanagh AC. 725 The human early pregnancy factor/chaperonin 10 gene family. Biochem Mol Med 1996; 58:52-58.
- Szenci O, Beckers JF, Humblot P, Sulon J, Sasser G, Taverne MAM, Varga J, Baltusen R, Schekk G. Comparison of ultrasonography, bovine pregnancy-specific protein B, and bovine pregnancy-associated glycoprotein 1 tests for pregnancy detection in dairy cows. Theriogenology 1998; 50:77-88.
- Thatcher WW, Guzeloglu A, Mattos R, Binelli M, Hansen TR, Pru JK. Uterine-conceptus interactions and reproductive failure in cattle. Theriogenology. 2001; 56:1435-1450.
- Thomas CM, Segers MF, Leloux AM, Houx PC. Comparison of the analytical characteristics of ten urinary hCG tests for early pregnancy diagnosis. Annals of Clinical Biochemistry. 1986; 23:216-222.
- Thompson JA, Marsh WE, Calvin JA, Etherington WG, Momont HW, Kinsel ML. Pregnancy attrition associated with pregnancy testing by rectal palpation. J Dairy Sci 1994; 77:3382-3387.
- Threlfall WR. Immunosuppressive early pregnancy factor (ISEPF) determination for pregnancy diagnosis in dairy cows. Theriogenology 1994; 41:317 (Abstr).
- Thurmond MC, Picanso JP. Fetal loss associated with palpation per rectum to diagnose pregnancy in cows. J Am Vet Med Assoc 1993; 203:432-435.
- Vailes LD, Britt JH. Influence of footing surface on mounting and other sexual behaviors of estrual Holstein cows. J Anim Sci 1990; 68:2333-2339.
- Vaillancourt D, Vierschwal CJ, Ogwu D, Elmore RG, Martin CE, Sharp AJ, Youngquist RS. Correlation between pregnancy diagnosis by membrane slip and embryonic mortality. J Am Vet Med Assoc 1979; 175:466-468.
- Vasconcelos JLM, Silcox RW, Lacerda JA, Pursley JR, Wiltbank MC. Pregnancy rate, pregnancy loss, and response to heat stress after AI at two different times from ovulation in dairy cows. Biol Reprod 1997; 56(Suppl 1):140 (Abstr).
- Weibold JL. Embryonic mortality and the uterine environment in first-service lactating dairy cows. J Reprod Fertil 1988; 84:393-395.
- White ME, LaFaunce N, Mohammed HO. Calving outcomes for cows diagnosed pregnant or nonpregnant by per rectum examination at various intervals after insemination. Can Vet J 1989; 30:867-870.
- Wimpy TH, Chang CF, Estergreen VL, Hillers JK. Milk progesterone enzyme immunoassay: modifications and a field trial for pregnancy detection in dairy cows. J Dairy Sci 1986; 69:1115-1121.
- Wisnicky W, Cassida LE. A manual method for diagnosis of pregnancy in cattle. J Am Vet Med Assoc 1948; 113:451.
- Wolf E, Arnold GJ, Bauersachs S, Beier HM, Blum H, Einspanier R, Frohlich T, Herrler A, Hiendleder S, Kolle S, Prelle K, Reichenbach HD, Stojkovic M, Wenigerkind H, Sinowatz F. Embryo-maternal communication in bovine – strategies for deciphering a complex cross-talk. Reprod Dom Anim 2003; 38:276-89.
- Wooding FBP. Current topic: the synepitheliochorial placenta of ruminants: binucleate cell fusions and hormone production. Placenta 1992; 13:101-113.
- Xie S, Green J, Bixby JB, Szafranska B, DeMartini JC, Hecht S, Roberts RM. The diversity and evolutionary relationships of the pregnancy-associated glycoproteins, an aspartic proteinase subfamily consisting of many trophoblast-expressed genes. Proc Natl Acad Sci USA 1997; 94:12809-12816.
- Xie S, Low BG, Kramer KK, Nagel RJ, Anthony RV, Zoli AP, Beckers JF, Roberts RM. Identification of the major pregnancy-specific antigens of cattle and sheep as inactive members of the aspartic proteinase family. Proc Natl Acad Sci USA 1991; 88:10247-10251.
- Zemjanis R. Diagnostic and therapeutic techniques in animal reproduction (2nd Ed.). Baltimore, Williams and Wilkins. 1970; pp 29-45.
- Zoli AP, Beckers JF, Wouters-Ballman P, Closset J, Falmagne P, Ectors F. Purification and characterization of a bovine pregnancy-associated glycoprotein. Biol Reprod 1991; 45:1-10.
- Zoli AP, Demez P, Beckers JF, Reznik M, Beckers A. Light and electron microscopic immunolocalization of bovine pregnancy-associated glycoprotein in the bovine placentome. Biol Reprod 1992b; 46:623-629.
- Zoli AP, Guilbault LA, Delahaut P, Ortiz WB, Beckers JF. Radioimmunoassay of a bovine pregnancy-associated glycoprotein in serum: its application for pregnancy diagnosis. Biol Reprod 1992a; 46:83-92.
Tables
| Number of pregnancies evaluated | Days of gestation at diagnosis | Loss interval, d | Pregnancy loss, % | Reference | |
|---|---|---|---|---|---|
| First | Second | ||||
| 256 | 28 | 38-58 | ~20 | 28.0 | Cartmill et al. (2001a) |
| 110 | 27-30 | 40-50 | ~16 | 42.7 | Cartmill et al. (2001b) |
| 195 | 28 | 42 | 14 | 17.9 | Chebel et al. (2003) |
| 89 | 28 | 56 | 28 | 13.5 | Fricke et al. (1998) |
| 209 | 26 | 68 | 42 | 27.8 | Fricke et al. (2003) |
| 77 | 33 | 68 | 35 | 11.7 | Fricke et al. (2003) |
| 139 | 27 | 45 | 18 | 20.7 | Moreira et al. (2001) |
| 172 | 28 | 45 | 17 | 9.3 | Santos et al. (2001) |
| 372 | 31 | 45 | 14 | 11.4 | Santos et al. (2001) |
| 215 | 27 | 41 | 14 | 9.9 | Santos et al. (2004b) |
| 705 | 28 | 42 | 14 | 3.2 | Silke et al. (2002) |
| First day detected | ||
|---|---|---|
| Characteristic | Mean | Range |
| Embryo proper | 20.3 | 19 to 24 |
| Heartbeat | 20.9 | 19 to 24 |
| Allantois | 23.2 | 22 to 25 |
| Spinal cord | 29.1 | 26 to 33 |
| Forelimb buds | 29.1 | 28 to 31 |
| Anmion | 29.5 | 28 to 33 |
| Eye orbit | 30.2 | 29 to 33 |
| Hindlimb buds | 31.2 | 30 to 33 |
| Placentomes | 35.2 | 33 to 38 |
| Split hooves | 44.6 | 42 to 49 |
| Fetal movement | 44.8 | 42 to 50 |
| Ribs | 52.8 | 51 to 55 |
| PAG RIA | Rectal palpation | Total | |
|---|---|---|---|
| Pregnant | Nonpregnant | ||
| Pregnant | 267 (93.03%) | 20 (6.97%) | 287 |
| Nonpregnant | 3 (2.10%) | 140 (97.90%) | 143 |
| Total | 270 | 160 | 430 |
| 1Numbers in parentheses indicate accuracy; total accuracy was 94.65%. | |||
| Test | Reader | Sensitivity2 | Specificity3 | PPV4 | NPV5 | Accuracy6 |
|---|---|---|---|---|---|---|
| 1 | 1 | 89% | 6% | 50% | 33% | 49% |
| (16/18) | (1/17) | (16/32) | (1/3) | (17/35) | ||
| 2 | 78% | 0% | 45% | 0% | 40% | |
| (14/18) | (0/17) | (14/31) | (0/4) | (14/35) | ||
| 2 | 1 | 94% | 0% | 50% | 0% | 49% |
| (17/18) | (0/17) | (17/34) | (0/1) | (17/35) | ||
| 2 | 83% | 12% | 50% | 40% | 49% | |
| (15/18) | (2/17) | (15/30) | (2/5) | (17/35) | ||
| Pooled results | 86% | 4% | 49% | 23% | 46% | |
| (62/72) | (3/68) | (62/127) | (3/13) | (65/140) | ||
|
1Serum samples from pregnant (n=18) and nonpregnant (n=17) dairy cattle were evaluated using two ECF test cassettes (Test 1 and 2) and the result of each test was interpreted by two independent readers (Readers 1 and 2). 2Proportion of serum samples from pregnant cattle with a positive ECF test result. 3Proportion of serum samples from nonpregnant cattle with a negative ECF test result. 4Probability that a positive ECF test result is from a pregnant animal. 5Probability that a negative ECF test result is from a nonpregnant animal. |
||||||
| Sunday | Monday | Tuesday | Wednesday | Thursday | Friday | Saturday |
|---|---|---|---|---|---|---|
| week 1 | PGF | |||||
| week 2 | ||||||
| week 3 | PGF | |||||
| week 4 | ||||||
| week 5 | GnRH | |||||
| week 6 | PGF | GnRH + TAI | ||||
| week 7 | ||||||
| week 8 | ||||||
| week 9 | ||||||
| week 10 | GnRH | |||||
| week 11 | PG+PGF | GnRH + TAI | ||||
| PGF = prostaglandin F2α, GnRH = gonadotropin-releasing hormone, TAI = timed artificial insemination, PG = pregnancy diagnosis using transrectal ultrasonography. | ||||||
| Item | Treatment group | Overall | ||
|---|---|---|---|---|
| D19 | D26 | D33 | ||
| Interval from Ovsynch TAI to 1st pregnancy exam (d) | 26 | 26 | 33 | – |
| PR/AI at 1st pregnancy exam, % | 46a | 42a | 33b | 40 |
| (no./no.) | (108/235) | (101/240) | (77/236) | (286/711) |
| Interval from Ovsynch TAI to 2nd pregnancy exam (d) | 68 | 68 | 68 | – |
| PR/AI at 2nd pregnancy exam, % | 33 | 30 | 29 | 31 |
| (no./no.) | (78/235) | (73/240) | (68/236) | (219/711) |
| Interval between pregnancy exams (d) | 42 | 42 | 35 | – |
| Pregnancy loss, % | 28a | 28a | 12b | 23 |
| (no./no.) | (30/108) | (28/101) | (9/77) | (67/286) |
| a,bWithin a row, percentages with different superscripts differ (P < 0.01) among treatment groups. | ||||
| Item | Treatment group | Overall | ||
|---|---|---|---|---|
| D19 | D26 | D33 | ||
| Mean (± SEM) interval (d) from Resynch | 27.1 ± 0.4 | 26.6 ± 0.2 | 33.7 ± 0.4 | – |
| TAI to pregnancy exam (range) | (26 to 54) | (26 to 40) | (26 to 75) | |
| PR/AI, % | 23a | 34b | 38b | 32 |
| (no./no.) | (28/120) | (41/121) | (54/143) | (123/384) |
| a, bWithin a row, percentages with different superscripts differ (P < 0.01) among treatment groups. | ||||
Figures


Author
Paul M. Fricke
University of Wisconsin, Madison, WI, USA
