Summary
Maximizing milk production without incurring ruminal acidosis is a challenge for most dairy producers. Feeding a highly fermentable diet provides energy precursors needed for high milk production, but the risk of subacute ruminal acidosis (SARA) increases. Ruminal acidosis is characterized by periodic episodes of suboptimal rumen pH, which depresses fiber digestion and possibly milk production. Preventing SARA requires careful management of rumen fermentation. Key strategies that help reduce the risk of acidosis are adaptation of the rumen environment to changes in diet composition, formulation of diets with slow rate of ruminal carbohydrate digestion, and increased intake of physically effective fiber. New research developments are improving our understanding of the factors that put cows at risk of developing SARA and how this risk can be managed.
Please check this link first if you are interested in organic or specialty dairy production.
Introduction
There is increasing concern about the prevalence of SARA in dairy cows, and several excellent reviews have been published (e.g., Krause and Oetzel, 2006; Enemark, 2008). Subacute ruminal acidosis is an increasing problem for the dairy industry, even in well-managed, high-yielding dairy herds. The reality is that some occurrence of SARA is inevitable in most high-producing dairy cows, given their high level of dry matter intake (DMI) and the high proportion of grain included in lactation diets. It is crucial to develop an understanding of the factors that put cows at risk of developing SARA and how feeding and management practices can help minimize this risk.
Defining Ruminal Acidosis
Acute Acidosis
Ruminal acidosis in cattle can be defined as acute or subacute. During acute ruminal acidosis, the pH in the rumen drastically drops (< 4.8) and remains low for an extended period of time (> 24 h). Intervention is usually needed to reverse acute acidosis. If left unattended, acute acidosis can develop into systemic (e.g., metabolic) acidosis (Owens et al., 1998). It is caused mainly by a buildup of lactic acid in the rumen, which usually results from an abrupt increase in the intake of rapidly fermentable carbohydrates. Clinical signs of acute acidosis include complete anorexia, abdominal pain, rapid beating of the heart, abnormally fast breathing, diarrhea, lethargy, and eventually death (Krause and Oetzel, 2006). Cattle that experience acute acidosis often become “poor doers” due in part to damage of the gastrointestinal tract. Fortunately, the prevalence of acute acidosis in dairy herds is very low.
Subacute Ruminal Acidosis (SARA)
Subacute ruminal acidosis is characterized by repeated bouts of low rumen pH, but unlike the situation with acute acidosis, the pH recovers after each bout. These bouts of low pH typically last for several minutes or several hours. The long bouts (> 3-4 h) are of concern because they negatively affect fiber digestion (Russell and Wilson, 1996), decrease the absorptive capacity of the ruminal epithelium (Harmon et al., 1985), and even damage the rumen epithelium. Damage to the absorptive tissues within the gastrointestinal tract increases the potential for bacteria, amines, and the toxins produced by bacteria (lipopolysaccharides) to enter the portal circulation, causing liver abscesses and an inflammatory response (Gohzo et al., 2005). There is increasing evidence that these toxins are implicated in laminitis (Mungall et al., 2001).
Subacute acidosis is caused by the accumulation of volatile fatty acids (VFA) in the rumen. As feed is digested, VFA (acetate, propionate, butyrate) are produced. The pH in the rumen drops if VFA production is rapid and exceeds the capacity of the rumen to maintain equilibrium. With time, the VFA are absorbed, buffered, or passed from the rumen, causing the pH to rise. As a consequence, a cyclical pattern of pH occurs causing periods of SARA. During SARA, lactic acid concentrations remain very low (< 1 mM).
An example of a pH tracing for a lactating dairy cow experiencing SARA is shown in Figure 1. The pH tracing was obtained using an indwelling ruminal pH monitoring system (Penner et al., 2006). The time that pH remained below the threshold pH value of 5.8 in each 24-h period was used to characterize SARA. Subacute ruminal acidosis occurred for 6.4, 6.5, and 11.8 h/d on Aug 5/6, 6/7, and 7/8, respectively. While indwelling ruminal pH monitoring systems are widely used in research, there is no reliable system for continuously measuring ruminal pH in commercial dairy cows. The limitation to developing such a system is the need to regularly standardize the pH electrodes to avoid drifting. Because the electrodes need to be retrieved for standardization, this technology is limited to use in ruminally cannulated cattle.
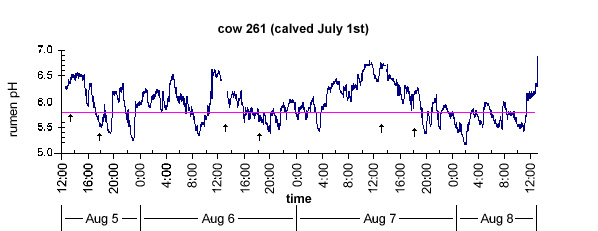
Fig. 1. Ruminal pH measured in a dairy cow over a 72-h period. Arrows show feeding times at 1330 and 1600 h; the solid line indicates the ruminal acidosis threshold of pH 5.8.
Lameness, diarrhea, and low milk fat percentage are often problems in herds with SARA, but these can also be caused by other problems. Individual cows with SARA do not necessarily show any clinical signs. Thus, without directly measuring rumen pH, it is difficult to identify animals suffering from SARA. Rumenocentesis (i.e., a small needle is used to take fluid from the ventral rumen) is sometimes used to detect herds with SARA, but a spot sampling approach has obvious limitations. The percentage of cows ruminating at any given time has been considered by some to indicate herd rumen health. Many dairy nutritionists consider a dairy herd to have healthy rumen function when at least 40% of the cows are ruminating at any given time, other than when they are eating or being milked (Eastridge, 2000; Maekawa et al., 2002). Our research would suggest that having 40% of the cows ruminating at any one time is an adequate goal, but that it is not possible to accurately detect SARA via a decreased percentage of cows ruminating by making a single observation of a herd. Adequate detection requires that numerous observations (several days, and various times within the day) be taken to accurately estimate the percentage of cows ruminating within a herd. Furthermore, it is only possible to detect whether SARA is prevalent within a herd, which does not isolate specific cows with SARA. It is possible that developments in technology will someday allow for the automatic capture of behavior on commercial dairy farms, making detection of SARA through changes in rumination behavior a possibility in the future.
Animal Variability
The risk for acidosis is not the same for all cows. Individual dairy cows exhibit tremendous variation in the degree of acidosis they experience, even when fed and managed similarly. In a recent experiment, we induced SARA in lactating dairy cows by offering them a single meal of 4 kg of ground grain after a 12-h period of feed restriction (Dohme et al., 2008). Four cows were considered to be at high risk for acidosis because they were already experiencing mild SARA due to the fact that they were in early lactation and fed a high-concentrate diet (45% forage, DM basis); the other four cows were considered to be at low risk for acidosis because they were later in lactation and fed a higher-forage diet (60% forage, DM basis). All cows consumed the grain challenge, but the time that pH remained below 5.8 varied between 0 and 13 h for the low-risk cows and between 11 and 23 h for the high-risk cows. It is clear that cows that were at greater acidosis risk before the challenge experienced more severe acidosis. Thus, reducing the risk factors for acidosis helps reduce the severity of acidosis should it occur. Not much is known about what causes animal variation, but presumably it is related to the combined effects of level of feed intake, eating rate, sorting of feed, salivation rate, the inherent ruminal microbial population, previous exposure to acidosis, rate of passage of feed from the rumen, and other aspects of physiology and behavior.
A study (Penner et al., unpublished) has provided evidence that differences in the absorptive capacity of the ruminal epithelia may account for a large proportion of the variation in ruminal pH among animals. In that study, 24 sheep were used as a model to study acidosis using an acidosis challenge protocol. Sheep were randomly assigned to the control or SARA treatment and orally dosed with water or 5 g glucose/kg BW (2.2 M glucose solution), respectively. Three hours after the oral drench, sheep were euthanized and ruminal tissue was collected to measure the tissue uptake of acetate and butyrate in Ussing chambers (an in vitro system used to measure absorption of compounds across biological membranes). To gain an understanding into animal variability in ruminal pH, sheep in the SARA treatment group were classified as non-responders or responders based on the severity of SARA they experienced following the glucose drench. Interestingly, the absorption of acetate and butyrate did not differ between control sheep and responders, indicating that the mild episode of SARA did not affect epithelial function. However, non-responders had more than twice the acetate uptake and 64% greater butyrate uptake than responders. Regression analysis further confirmed that animals with increased absorption had higher ruminal pH (see Figure 2).
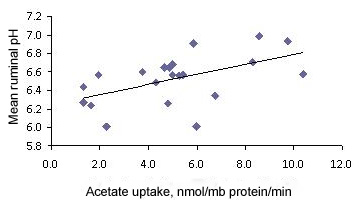
The bottom line is that cows vary markedly in their ability to cope with the dietary factors that predispose them to acidosis (Schwartzkopf-Genswein et al., 2003). This individuality makes it nearly impossible to completely eliminate acidosis from occurring in high-producing dairy herds, especially if diets are formulated for the average cow.
Effects of Repeated Episodes of Acidosis
To study the effects of chronic cases of SARA, we used a challenge model (as described previously) to induce SARA in dairy cows at high and low risk for acidosis (Dohme et al., 2008). We conducted three consecutive challenges, with each challenge spaced 14 d apart. The grain challenge was consumed in its entirety by all cows during the first challenge, but only 75% of the cows consumed the entire challenge in Period 2, and only 43% of the cows consumed the entire grain challenge in Period 3. Thus, the cows “learned” quickly to avoid the negative effects of overconsumption. Despite a learned aversion, the bout of SARA experienced with each challenge was increasingly severe. After the first challenge, the ruminal pH fully recovered within two days, but full recovery did not, however, occur after the second and third challenges (Figure 3). Failure of the pH to rebound may have been due to a destabilized ruminal microflora (Nagaraja and Titgemeyer, 2007). Also, damage to the rumen epithelium may have decreased its absorptive capacity (Harmon et al., 1985). Krehbiel et al. (1995) showed that a short-term, severe insult of acute acidosis decreased ruminal VFA absorption for an extended period of time. The bottom line is that once cows experience a bout of acidosis, they become increasingly susceptible to re-experiencing acidosis, and it is not clear whether they ever fully recover.

Increased Risk of Acidosis in Fresh Cows
Fresh cows are particularly vulnerable to acidosis because of the fairly abrupt change in fermentable carbohydrate intake that occurs after parturition. Gröhn and Bruss (1990) observed that the number of cases of ruminal acidosis was greatest during the first months after calving. Fairfield et al. (2007) reported that SARA increased dramatically after parturition in dairy cows fed a lactation diet consisting of 54% forage DM. During the first week after calving, mean pH averaged 6.19 and time below pH 6.0 was 7.3 h/d, whereas during the sixth week after calving, these were 6.36 and 3.4 h/d, respectively. We studied the occurrence of acidosis in primiparous cows fed a close-up diet, followed by a lactation diet containing adequate physically effective fiber (peNDF) (Penner et al., 2007). Mean ruminal pH dropped abruptly from an average of 6.32 before calving, to an average of 5.98 after calving (Table 1). In the first 40 days after calving, rumen pH was < 5.8 for 7 to 9 h/d, with 71 to 79% of the cows experiencing SARA. This study shows that primiparous cows are particularly susceptible to developing acidosis after parturition. Furthermore, from our previous studies, we have shown that if cows experience acidosis, they become more susceptible to subsequent bouts of acidosis. This would have long-term effects on health and productivity of these primiparous cows.
| Variable | Day relative to parturition | ||||
|---|---|---|---|---|---|
| -5 to -1 | 1 to 5 | 17 to 19 | 37 to 40 | 58 to 60 | |
| Minimum pH | 5.74a | 5.38b | 5.37b | 5.32b | 5.37b |
| Mean pH | 6.32a | 5.96b | 5.95b | 5.96b | 6.03b |
| SARA ( pH < 5.8), h/d | 1.1c | 7.3ab | 9.0a | 8.3ab | 6.1b |
| Proportion of cows experiencing SARA, % | 5 | 71 | 71 | 79 | 64 |
| abcP < 0.05. | |||||
Regulation of Ruminal pH
The digestion of large quantities of highly fermentable feeds leads to rapid production of VFAs in the rumen. Volatile fatty acids in the rumen occur both in the protonated (undissociated, H-VFA) and the ionized state (dissociated, VFA–) and absorption through the rumen epithelium is the main mechanism by which the VFA are removed from the rumen. Elevation of pH due to VFA absorption occurs as a result of clearance of the acid from the rumen during passive diffusion of the protonated VFA and by the neutralization of acid within the rumen when ionized VFA are absorbed in exchange for bicarbonate.
At high pH, most of the VFA are in the ionized state, whereas the opposite is true at low pH (< 6). Absorption of protonated VFA (H-VFA) by the rumen epithelium occurs passively. This process helps stabilize ruminal pH because the acid associated with the VFA is removed from the rumen. The rate of absorption of protonated VFA increases as the pH decreases, but there is likely a threshold at which decreased ruminal pH no longer increases the rate of passive diffusion due to damage to the rumen mucosa (Gäbel et al., 1989). Absorption of ionized VFA (VFA–) by the rumen epithelium occurs in exchange for bicarbonate, which can be from blood and from de novo synthesis within the epithelial cells (Gäbel et al., 2002). For every 1 mole of VFA– absorbed, 0.5 moles of bicarbonate are released. Thus, the ruminal wall is a potent source of bicarbonate. In an unpublished study by Penner et al., acetate absorption in the ionized state (i.e., associated with bicarbonate secretion) was significantly correlated to ruminal pH, but the passive diffusion of acetate was not correlated. In addition to pH of the rumen, the type of VFA (acetate, propionate, and butyrate) affects the way that VFA are absorbed from the rumen. In an in vitro study conducted at pH 6.1, Penner et al. (unpublished) demonstrated that only about 28% of acetate absorption was absorbed through passive diffusion, whereas about 72% of butyrate absorption was through passive diffusion. This indicates that the type of VFA may alter the ruminal buffering mechanism (i.e., the amount of acid removed through absorption or neutralized due to the secretion of bicarbonate coupled with VFA absorption).
Understanding the mechanisms involved in VFA absorption will improve our ability to formulate diets to manage rumen pH, which is especially important for cows in early lactation. Absorption of VFA from the rumen (removing acids, adding bicarbonate) is the major factor moderating ruminal pH. However, hydrogen ions are also removed from the rumen by the buffering effects of saliva, feed, and some feed degradation products (e.g., ammonia). Of these, saliva is by far the most significant source of buffer. Saliva contains bicarbonate and phosphate buffers that play a major role in buffering acids within the rumen. The implications of diet formulation on salivary secretion are discussed later in the paper.
Managing Ruminal VFAs – The Key to Preventing Acidosis
Adaptation Strategies
The rumen ecosystem is amazingly resilient to changes in diet, but the key is adaptation and stability. Rumen health is maintained by avoiding abrupt dietary changes and by adapting the rumen environment prior to calving. A sudden change in the amount of fermentable carbohydrates consumed by the cow can cause acidosis. Transition programs during the close-up period can help reduce the risk of acidosis postpartum. Transition programs must be designed to allow the rumen environment, including both the ruminal epithelium and the rumen microbial populations, to adapt to dietary changes. Gradual transition of cows from a high-forage far-away dry-cow diet to a lactation diet containing substantial quantities of grain helps stabilize microbial populations. The key is to ensure that lactic acid concentrations in the rumen remain low. Growth of ruminal bacteria that use lactic acid, e.g., Megasphaera elsdenii, Propionibacterium spp. is relatively slow; thus, these bacteria need sufficient time to adapt to dietary change (Nagaraja and Titgemeyer, 2007). In adapted cows, these bacteria ensure that any lactic acid produced is rapidly metabolized, thereby preventing lactic acid from accumulating in the rumen.
Feeding a close-up diet that is intermediate in fermentability between the far-away dry diet and the lactation diet stimulates the growth of the rumen epithelium, which increases the surface area for VFA absorption. It is thought that increasing the absorptive surface of the rumen helps prevent the accumulation of VFA in the rumen, the main driver of ruminal pH depression. The rumen papillae shorten during the dry period due to the low intake of fermentable carbohydrate and the reduced need for VFA absorption (Dirksen et al., 1985). During the close-up period, the papillae lengthen as cows are exposed to more grain in their diet. It can take up to eight weeks for maximum papillae growth to occur when animals are changed from a forage diet to a concentrate diet (Dirksen et al., 1985).
An unpublished study from our lab indicates that under North American feeding conditions, regression of papillae during the dry period is only moderate. Absorptive surface of the papillae during the far-away dry period is about 75% of what it is during lactation. Furthermore, feeding a close-up transition diet prepartum restored the surface area within two to three weeks. However, even though the absorptive surface area of the rumen was restored prepartum, cows experienced SARA postpartum. We concluded that the high incidence of SARA was due to the depression in feed intake that occurs as calving approaches. The ruminal epithelium regresses rapidly in response to decreased nutrient supply. For example, sheep fed a hay diet ad libitum had greater rates of VFA absorption than sheep fed the same diet but subjected to 48 h of feed withdrawl (Gäbel et al., 1993; Gäbel and Aschenbach, 2002). During the transition period, the characteristic reduction in DMI as calving approaches may negatively impact rumen epithelial function, without corresponding changes to the absorptive surface area, thereby increasing the susceptibility to SARA. As such, strategies to minimize the depression in feed intake as calving approaches may help reduce the severity of ruminal acidosis following calving. The amount of time required to restore epithelial function has not been examined and deserves attention from future research. We conclude that it is the functionality of the rumen epithelium rather than the papillae surface area itself that helps protect the cow from accumulating VFA in the rumen. A better understanding of the cellular mechanisms of VFA absorption will help us improve recommendations for transition diets.
Decreasing the Rate of Fermentation of the Diet
The amount of substrate fermented in the rumen drives VFA production. Thus, high intakes of rapidly digestible feeds such as grains and high-quality forages result in the rapid production of VFA and increase the risk of SARA. In particular, starch and sugar, which are more rapidly digested in the rumen than structural carbohydrates, increase the risk of SARA. Cereal grains are the main source of starch in the cow’s diet, although grain silages also contribute significantly to starch intake. The structure and morphology of the various grains determine their susceptibility to ruminal digestion. In general, the rate of ruminal starch digestion is as follows: wheat > oats > barley > corn > sorghum. While there are inherent differences among the types of cereal grains in their ruminal digestibilities, processing can alter the rate of ruminal digestion of all cereal grains. High-moisture corn is highly fermentable in the rumen, and with dry corn, the rate of digestion increases with heat treatment (steam-rolling, steam-flaking) and with decreased particle size (Callison et al., 2001).
The risk of SARA increases as the rate of starch digestion increases. To illustrate this concept, Krause et al. (2002) compared the effects of feeding high-moisture corn or dry cracked corn to dairy cows (Figure 4). Even though particle size of the forage was coarse, rumen pH was lower for cows fed high-moisture corn because of its higher fermentability. At the same concentration of peNDF, diets will result in lower rumen pH when they contain higher levels of nonfiber carbohydrates and/or carbohydrates that are rapidly digested.

Fig. 4. Ruminal pH of dairy cows fed high-moisture corn (HMC) versus cracked shelled corn (DC). The forage was coarsely chopped (CS) corn silage (Krause et al., 2002).
One approach to slowing the rate of fermentation is to lower the starch and sugar content of the diet. We recommend a maximum of 28% starch, 6% sugar, and 35 to 40% nonfiber carbohydrates in lactation diets. Replacing a portion of the grain with nonforage sources of fiber such as beet pulp, soybean hulls, alfalfa meal, distillers dried grains, brewers grains, and corn gluten feed can help limit the starch content of the diet (Grant, 1997). However, the most effective strategy to slowing fermentation rate in the rumen is to increase the proportion of forage in the diet because forage is digested much more slowly than grain. Adding forage to the diet tends to even out VFA production and minimize the large fluctuations in rumen pH that can occur after meals.
Providing Physically Effective Fiber (peNDF)
The peNDF content of the diet can be increased either by 1) increasing the NDF content (i.e., by including more forage), or 2) increasing the chop length of forages. Either way of increasing the peNDF content of the diet increases chewing time and salivary secretion. However, feeding a higher proportion of forage to increase the dietary NDF content has the added benefit of lowering the starch concentration of the diet, which slows the rate of fermentation of the diet. Thus, increasing the peNDF content of the diet by increasing the NDF content is usually more effective in terms of preventing SARA than is increasing forage particle length (Yang and Beauchemin, 2007).
Regardless of whether peNDF is increased through forage proportion or particle size, increasing peNDF intake reduces the risk of SARA. Mean ruminal pH and the duration that pH remains below 5.8 are highly correlated to intake of long particles, measured as particles retained on the 19-mm sieve of the Penn State Particle Separator (PSPS), or the total amount retained on the 19-mm and the 8-mm sieves of the PSPS (Yang and Beauchemin, 2007). To minimize SARA in lactating cows, we recommend a minimum of 19 to 21% NDF from forage sources. For the forages, 60% or more of the material should be retained on the top two sieves. However, it is undesirable to have more than 15% of the particles on the top sieve because those particles cause increased sorting by cows.
With diets low in peNDF, each additional kilogram of peNDF will increase chewing time by up to 7 h/d. With diets containing adequate peNDF, each additional kilogram of peNDF promotes 0 to 2 h/d of additional chewing. Thus, a small increase in the peNDF content of the diet can be very effective when diets are low in fiber but not when diets already contain adequate fiber levels. Even though increasing the peNDF content of the diet increases chewing time, the increase in saliva output due to increased chewing is not as great as often assumed. This is because the increased flow of saliva during chewing is accompanied by a decrease in resting saliva secretion. The net increase in total salivary secretion due to 1 h/d more chewing is about 7 L (Maekawa et al., 2002). The buffering capacity supplied by the additional saliva would adequately buffer the digestion of about 0.5 to 0.75 kg of ground grain. Thus, the net effect of this incremental saliva production on mean rumen pH is relatively small. It has been estimated that salivary buffers neutralize from 15% (Gäbel et al., 2002) to 40% (Allen, 1997) of the VFA produced in the rumen. However, an increase in saliva secretion, particularly if secreted during eating, can help reduce the extent to which pH drops below 5.8 following meals, even though mean rumen pH is not greatly affected. Thus, the reduction in SARA that occurs with increased peNDF intake is not directly related to increased chewing activity. Rather, the reduction in acidosis with increased peNDF intake is due to the combined effects of increased chewing activity, reduced meal size, slower eating rate, increased rumen motility, and a shift in the site of grain digestion.
Rumen Fermentation Modifiers
In addition to diet formulation, additives that help modify the microbial ecology of the rumen, such as sodium bicarbonate, yeast, bacterial direct-fed microbials, and monensin can help minimize acidosis. Information on most of these additives is given in a separate proceedings paper at this conference, so our comments here are limited to the use of buffers. Buffers are fairly routinely added to most lactating cow diets as a precautionary measure in the prevention of acidosis. We recommend adding sodium bicarbonate at 0.5 to 0.75% of DMI, introduced into the ration gradually due to their low palatability. Adding buffers to the diet is approximately equal to the net buffering effect of increasing the proportion of forage in the diet by 5% percentage units or 1 h/d more chewing time. When mixed into the ration, sodium bicarbonate can help elevate ruminal pH by buffering and by increasing the liquid dilution rate from the rumen, which increases the flow of starch from the rumen. Buffers are sometimes offered free choice to cattle based on the assumption that ruminants will readily consume the amount needed to attenuate the effects of SARA. We recently conducted a study where cattle experiencing SARA (pH was below 5.8 for more than 10 h/d) were provided with sodium bicarbonate, either mixed into the ration or offered free choice (Patton et al., 2006). Mixing the bicarbonate into the ration was more effective than providing it free choice because it reduced the number of long bouts of ruminal acidosis each day. Intake of free choice bicarbonate was highly variable among animals and from day to day, but this variability was not related to ruminal pH. It is possible that cattle may learn the benefits of consuming buffers over a long period of time, but as a precaution, buffers should be added directly to the ration.
Conclusions
Feeding dairy cows for maximum milk production increases the risk of acidosis, which can reduce efficiency of milk production and jeopardize cow health. Ruminal acidosis occurs when the rate of VFA production in the rumen exceeds the rate at which the rumen environment can neutralize or absorb the acids. Carefully adapting the rumen to changes in diet, supplying adequate peNDF concentration, and reducing the fermentability of the nonfiber carbohydrate fraction are the main factors to consider in preventing ruminal acidosis. Use of high-quality forages helps cushion against the risk of ruminal acidosis because a greater proportion of forage can be included in the diet without lowering its digestible energy content.
Most diets are formulated for the average cow and do not include a margin of error to account for the variability among cows. As diets are formulated closer to the minimum level of peNDF and with higher concentrations of nonfiber carbohydrates, a greater portion of the cows will experience ruminal acidosis. Formulating diets for the average cow may be acceptable for cows in mid- and late lactation, but diets for cows in early lactation need to account for the higher risk of these cows to experiencing acidosis.
Author Information
Karen A. Beauchemin, Agriculture and Agri-Food Canada
Greg Penner, University of Alberta
References
Allen, M.S. 1997. Relationship between fermentation acid production in the rumen and the requirement for physically effective fiber. J. Dairy Sci. 80: 1447-1462.
Callison, S.L., J.L. Firkins, M.L. Eastridge, and B.L. Hull. 2001. Site of nutrient digestion by dairy cows fed corn of different particle sizes or steam-rolled. J. Dairy Sci. 84:1458–1467.
Dirksen, G.U., H.G. Liebich, and E. Mayer. 1985. Adaptive changes of the ruminal mucosa and their functional and clinical significance. Bovine Pract. 20:116–120.
Dohme, F., T.J. DeVries, and K.A. Beauchemin. 2008. Repeated ruminal acidosis challenges in lactating dairy cows at high and low risk for developing acidosis: ruminal pH. J. Dairy Sci. 91: 3554-3567.
Eastridge, M.L. 2000. Walking the rumen health tightrope. Hoard’s Dairyman. Vol. 145, p. 626. Fort Atkinson, Wis.
Enemark, J.M.D. 2008. The monitoring, prevention and treatment of subacute ruminal acidosis (SARA): A review. The Veterinary Journal 176:32–43.
Fairfield, A.M., J.C. Plaizier, T.F. Duffield, M.I. Lindinger, R. Bagg, P. Dick, and B.W. McBride. 2007. Effects of prepartum administration of a monensin controlled-release capsule on rumen pH, feed intake, and milk production of transition dairy cows. J. Dairy Sci. 90:937–945.
Gäbel, G., M. Marek, and H. Martens. 1991. Influences of diet, short-chain fatty acids, lactate and chloride on bicarbonate movement across the reticulo-rumen wall of sheep. J. Vet. Med. A. 38:523-529.
Gäbel, G., M. Marek, and H. Martens. 1993. Influence of food deprivation on SCFA and electrolyte transport across sheep reticulorumen. Zentbl. Vetmed. Reihe A 40:339–344.
Gäbel, G., J.R. Aschenbach, and F. Müller. 2002. Transfer of energy substrates across the ruminal epithelium: implications and limitations. Animal Health Research Reviews 3:15–30.
Gäbel, G., and J.R. Aschenbach. 2002. Influence of food deprivation on the transport of 3-O-methyl-ɑ-D-glucose across the isolated ruminal epithelium of sheep. J. Anim. Sci. 80:2740-2746.
Gaebel, G., M. Bell, and H. Martens. 1989. The effect of low mucosal pH on sodium and chloride movement across the isolated rumen mucosa of sheep. Q. J. Exp. Physiol. 74:35–44.
Grant, R.J. 1997. Interactions among forages and nonforage fiber sources. J. Dairy Sci. 80:1438–1446.
Gozho, G.N., J.C. Plaizier, D.O. Krause, A.D. Kennedy, and K.M. Wittenberg. 2005. Subacute ruminal acidosis induces ruminal lipopolysaccharide endotoxin release and triggers an inflammatory response. J. Dairy Sci. 88:1399–1403.
Gröhn, Y.T., and M.L. Bruss. 1990. Effect of diseases, production, and season on traumatic reticuloperitonitis and ruminal acidosis in dairy cattle. J. Dairy Sci. 73:2355–2363.
Harmon, D.L., R.A. Britton, R.L. Prior, and R.A. Stock. 1985. Net portal absorption of lactate and volatile fatty acids in steers experiencing glucose-induced acidosis or fed a 70% concentrate diet ad libitum. J. Anim. Sci. 60:560-569.
Krause, K.M., D.K. Combs, and K.A. Beauchemin. 2002. Effects of forage particle size and grain fermentability in mid-lactation cows. II. Ruminal pH and chewing activity. J. Dairy Sci. 85:1947–1957.
Krause, M.K., and G.R. Oetzel. 2006. Understanding and preventing subacute ruminal acidosis in diary herds: a review. Anim. Feed Sci. Technol. 126:215–236.
Krehbiel, C. R., R.A. Britton, D.L. Harmon, T.J. Wester, and R.A. Stock. 1995. The effects of ruminal acidosis on volatile fatty acid absorption and plasma activities of pancreatic enzymes in lambs. J. Anim. Sci. 73:3111–3121.
Maekawa, M., K.A. Beauchemin, and D.A. Christensen. 2002. Effect of concentrate level and feeding management on chewing activities, saliva secretion, and ruminal pH of lactating dairy cows. J. Dairy Sci. 85:1165–1175.
Mungall, B.A., M. Kyaw-Tanner, and C.C. Pollitt. 2001. In vitro evidence for a bacterial pathogenesis of equine laminitis. Vet. Microbiol. 79:209-223.
Nagaraja, T.G., and E.C. Titgemeyer. 2007. Ruminal acidosis in beef cattle: The current microbiological and nutritional outlook. J. Dairy Sci. 90 (E. Suppl.):E17-E38.
Owens. F.N., D.S. Secrist, W.J. Hill, and D.R. Gill. 1998. Acidosis in cattle: a review. J. Anim. Sci. 76:275-286.
Paton, L.J., K.A. Beauchemin, D.M. Veira, and M.A.G. von Keyserlingk. 2006. Use of sodium bicarbonate, offered free choice or blended into the ration, to reduce the risk of ruminal acidosis in cattle. Can. J. Anim. Sci. 86:429–437.
Penner, G.B., K.A. Beauchemin, and T. Mutsvangwa. 2006. An evaluation of the accuracy and precision of a stand-alone submersible continuous ruminal pH measurement system. J. Dairy Sci. 89:2132–2140.
Penner, G.B., K.A. Beauchemin, and T. Mutsvangwa. 2007. Severity of ruminal acidosis in primiparous Holstein cows during the periparturient period. J. Dairy Sci. 90:365-375.
Russell, J.B., and D.B. Wilson. 1996. Why are ruminal cellulolytic bacteria unable to digest cellulose at low pH? J. Dairy Sci. 79:1503-1509.
Yang, W.Z., and K.A. Beauchemin. 2007. Altering physically effective fiber intake through forage proportion and particle length: Chewing and ruminal pH. J. Dairy Sci. 90:2826–2838.
