Summary
- The 60-d dry period was adopted as a management practice during World War II.
- Since that time, large production increases and advances in dairy management practices (i.e., bST, increased milking frequency, photoperiod management) warrant a reevaluation of optimal dry period length in dairy cows.
- Recent research on modified dry periods in dairy cows has demonstrated:
- Equal milk yields in 60-d and 30-d dry multiparous cows with or without bST supplementation.
- Equal milk yields in 60-d dry and bST-supplemented continuously milked (CM), multiparous cows.
- Modified dry periods negatively affect subsequent milk yield in primiparous cows.
- Primiparous cows continue to require a 60-d dry period.
- Modified dry periods may improve dry matter intake (DMI) and minimize extremes in metabolic and physiologic changes throughout late gestation and early lactation.
- Diet changes during the last 60 d of gestation are minimized to one or zero.
- DMI is improved in cows given short or no dry period.
- Mammary engorgement and involution at dry-off are lessened with short dry periods and eliminated with no dry period.
- No dry period or continuous milking keeps animals coordinated for lactation, which reduces metabolic and physiologic changes associated with the onset of lactation at parturition.
Please check this link first if you are interested in organic or specialty dairy production
History of the 60-Day Dry Period
The optimal dry period length between lactations in dairy cows has been debated since the early 1800s (Dix Arnold and Becker, 1936). During this time, some English farmers believed that a two-month dry period was optimal, while others believed that a two-week dry period was adequate. More than a century later during World War II, the 60-d dry period was adopted as the optimal dry period length for maximal milk yield and genetic progress during this time of food shortage (Knight, 1998). Since its adoption, the 60-d dry period has been maintained as the dry period length that best maintains the balance between lost milk income during the dry period and production levels achieved in the subsequent lactation. Currently, a majority of U.S. dairies manage for a 60-d or longer dry period (USDA, 2002). However, there have been many changes since adoption of the 60-d dry period that include a 5000 kg increase in milk yield per lactation, a larger emphasis on profit, accelerated genetic progress through artificial insemination and embryo transfer, adoption of the total mixed ration, increased milking frequency (IMF), altered photoperiod management, and commercialization and adoption of bovine somatotropin (bST) (Annen et al., 2004c). Such changes warrant a reevaluation of the optimal dry period length in today’s high-producing dairy cow.
Hypothesis for a Dry Period Requirement
There have been four hypotheses proposed for reduced milk yield in cows subjected to short or omitted dry periods. They include: 1) nutritional limitations during late-gestation, 2) hormonal differences, 3) reduced mammary epithelial cell (MEC) number, and 4) reduced synthetic and mitotic functionality of MEC (reviewed by Annen et al., 2004c).
The nutritional hypothesis suggested that the reduction in subsequent milk yield in cows subjected to modified dry periods was caused by decreased replenishment of body reserves during the last 60 d of gestation, thus resulting in inadequate body reserves to partition to milk production in the subsequent lactation. This hypothesis was disproved by studies demonstrating improved body weights but lower milk yields in continuously milked (CM) or 30-d dry cows compared to 60-d dry cows (Table 1) (Swanson et al., 1965; Lotan and Alder, 1976) and by a half-udder study demonstrating reduced milk yield in CM quarters despite equal nutritional factors to all quarters (Smith et al., 1967).
The hormonal hypothesis suggested the constant influence of galactopoietic and milking stimulus hormones associated with maintenance of lactation during the last 60 d of gestation caused reduced milk yields in the succeeding lactation. In a half-udder study, Smith et al. (1967) demonstrated reduced milk yield in CM quarters compared to 60-d dry quarters, although all quarters had equal exposure to endocrine hormones (Table 1). One aspect of the hormonal hypothesis that remains unanswered is the effect of CM or short dry periods on locally produced and/or acting hormones. Modified dry periods may alter or inhibit the actions of these hormones that work locally within the mammary gland. Collectively these experiments investigating nutritional and endocrine hypotheses suggested that negative effects of modified dry periods were within the mammary gland rather than on systemic factors regulating milk synthesis.
An understanding of the changes that the mammary gland undergoes during a traditional dry period is important when evaluating the effects of continuous milking on the mammary gland. Concurrent advanced pregnancy and lactation at the time of dry-off in dairy cows results in involution and the dry period being a process of MEC replacement and modest remodeling of the gland (Capuco and Akers, 1999). In species that are nonpregnant at dry-off or weaning of offspring, the mammary gland goes through a phase of extensive cell loss followed by gland remodeling to structural similarity to a virgin gland (Furth, 1999). Lactogenic and mammogenic stimuli of advanced pregnancy oppose apoptotic (cell death) stimuli induced by cessation of milking (Capuco et al., 2004). Therefore, in dairy cows extensive cell loss does not occur during involution, and MEC growth begins to increase before the involution process is complete (Annen et al., 2003). A transient and rapid increase in MEC apoptosis occurs during the first 72 h after dry-off, followed by initiation of MEC proliferation and decreased apoptosis within 7 to 10 d after dry-off (Annen et al., 2003). Mammary gland remodeling in dairy cows includes a reduction in luminal area of alveoli, an increase in stromal components, and synthesis of extracellular matrix (Holst et al., 1987; Hurley, 1989; Capuco et al., 1997). Capuco et al. (1997) demonstrated that the involution process is complete by 25 d dry and that MEC proliferation increased throughout the dry period. At completion of involution, the mammary gland of dairy cows is non-lactating but maintains structural competence for milk secretion (Annen et al., 2003; Holst et al., 1987).
The impact of continuous milking on mammary gland dynamics that normally occur during the dry period has been the focus of research investigating mammary
functionality in CM cows. In dairy cows, continuous milking (Capuco et al., 1997) or a six-week difference in dry period length (Swanson et al., 1967) did not alter total mammary DNA content (cell number) or parenchyma content during late gestation when compared to 60-d dry cows. Measures of mammary size by Fowler et al. (1991) suggested reduced cell number in CM goats at the time of parturition. In CM rats, total DNA was higher at parturition compared to non-lactating rats. Premature MEC loss in CM rats during early lactation resulted in lower total mammary DNA content than rats that were given a dry period (Paape and Tucker, 1969). Total mammary DNA, MEC numbers, and MEC loss during early lactation have not been investigated in CM dairy cows.
Reduced mammary functionality in CM cows was the hypothesis proposed to describe reductions in milk yield in CM cows, despite equal cell numbers (Swanson et al., 1967). Capuco et al. (1997) measured MEC proliferation indices in addition to total cell numbers and demonstrated that MEC proliferation was reduced in CM glands compared to non-lactating glands throughout the last 35 d of gestation. Maintenance of total cell number and parenchyma mass in the face of reduced MEC proliferation suggests increased carryover of old MEC into the next lactation rather than replacement of old MEC with new MEC as occurs in non-lactating glands during late gestation. Increased MEC carryover has been demonstrated in CM rats (Pitkow et al., 1972). Reductions in milk yield in cows subjected to modified dry periods are believed to be caused by larger populations of old MEC in CM glands compared to 60-d-dry glands. This hypothesis assumes that older MEC have decreased capacity for milk synthesis and reduced mitotic competence.
Production Results from Previous Research
Milk yield responses to planned (Table 1) and retrospective studies on modified dry periods have been recently reviewed by Bachman and Schairer (2003), Annen et al. (2004c), and Grummer and Rastani (2004). Results from planned experiments are summarized in Table 1. Retrospective studies, which are analyses of dairy records, will not be discussed. These studies evaluated the effects of short or omitted dry periods using records from herds that were managed for a 60-d or longer dry period. Reasons for short or no dry periods in these herds are unknown but are likely the result of multiple births, mismanagement, and aborted pregnancies, all factors that would negatively bias subsequent milk yield.
Production responses to shortened dry periods ranged from 0 to 10% reductions in subsequent milk yield (Table 1) (Coppock et al., 1974; Lotan and Alder, 1976; Sorensen and Enevoldsen, 1991). Production responses to omitted dry periods or continuous milking range from 17 to 38% reductions in subsequent milk yield (Table 1) (Swanson, 1965; Smith et al., 1967; Remond et al., 1992). Importantly, the animals used in these experiments achieved much lower peak milk yields than today’s high-producing cow. Peak milk yield in these studies was 18 to 34 kg/d compared to peak milk yields of 45 kg/d or more in today’s cow. Further, many modern dairy management practices were not utilized in these studies.
Opportunities for a Change in the Dry Period Requirement
Increased production levels and improved persistency of lactation in today’s dairy cow (Figure 1) provide new reasons to reevaluate the optimal dry period length in dairy cows.
The first reason is increased milk yield at the time of dry-off (Figure 1). Many cows are producing more than 30 kg/d at dry-off and have the potential to milk through the last 60 d of gestation at a profitable production level. Additional days of lactation maximize income generated per cow per lactation and decrease the number of replacement animals needed to keep a dairy operating at desired cow number capacity. While high yields at dry-off provide an economical reason for modified dry periods, they also create a cow health and comfort reason for short or omitted dry periods. High yields at dry-off result in extreme changes in metabolic and physiological state, which are complicated with dramatic diet changes. Such extremes result in the addition of a second transition period to the lactation cycle. For example, the cow undergoes the discomfort of udder engorgement and involution during the early dry period combined with a diet change from a lactating ration to a far-off dry cow ration as the animal and mammary gland go from producing 30 kg/d to 0 kg/d at dry-off.
Another reason to investigate modified dry periods in high-producing cows lies at the other end of the lactation curve in the “traditional” transition period (three-week prepartum to three-week postpartum) (Grummer, 1995) when the stress of parturition, initiation of lactation, and dramatic diet changes occur. These two transition periods within 60 d of each other offer the cow a relatively short time period to adapt to several acute changes in metabolic and physiologic states and diets. Continuous milking may keep the animal adapted to lactation and associated diets and reduce the incidence of metabolic disorders, as well as improve dry matter intake (DMI) during this transition period.
The third and perhaps most important reason to reevaluate optimal dry period length comes from new management technologies that were introduced since World War II that improve milk yield (Figure 1). Some of these management technologies, including IMF, bST, and photoperiod management, are known to increase milk yield and therefore may address the issue of reduced mammary functionality in MEC without a rest period between lactations.
Bovine somatotropin (bST) is of particular interest and will be the primary focus of following sections of this paper. Exogenous bST increases milk yield by 10 to 15% and improves lactation persistency (Bauman et al., 1999). Most importantly, bST has been shown to impact lactating MEC by all or a combination of the following mechanisms: 1) improved synthetic activity on a per cell basis (increased RNA content), 2) increased number of cells in a secretory state and reduced number of cells in a resting state (increased parenchyma volume without increased DNA synthesis), and 3) reduced cell loss (low levels of plasmin during bST treatment) (Bauman and Vernon, 1993). Capuco et al. (2001) recently reported an increase in MEC proliferation in bST-treated, lactating cows. However, the rate of MEC apoptosis did not change with proliferation, so the net effect was a reduced rate of mammary regression during the decline phase of lactation rather than net accumulation of mammary parenchyma. Additionally, bST has been demonstrated as mammogenic in non-lactating, pregnant heifers (Collier et al., 2002), but bST treatment during late pregnancy has not translated to increased milk yield in the ensuing lactation (Bachman et al., 1992; Collier et al., 2002). In ewes, bST treatment during the dry period enhanced mammary growth and postpartum milk yield (Stelwagen et al., 1993). The effects of bST in late-pregnant, lactating cows had not been investigated until recently and will be discussed below.
Recent Research on Modified Dry Periods
Recent research, some of which incorporates bST supplementation, IMF, and other mammogenic treatments, has demonstrated potential for reducing dry period length in high-producing cows. The 30-d dry period has been shown to be equal to a 60-d dry period with or without bST supplementation during late gestation and early lactation

(Bachman, 2002; Gulay et al., 2003; Rastani et al., 2003; Annen et al., 2004d). Altered MEC turnover and reduced milk yield in CM or short-dry-period cows were evaluated using a shortened dry period (30 d) and estrogen injection at milk stasis to attempt to hasten involution (Bachman, 2002). The 305-d ME was equivalent in 30-d dry and 60-d dry groups (Table 1). Estrogen treatment did not alter 305-d ME regardless of dry period length. The fact that mammary involution is complete by 25 d of the dry period (Capuco et al., 1997) suggests that a 30-d dry period is long enough for involution to occur without estrogen treatment. Additionally, these data suggest a 30-d dry period may also be an adequate length to alleviate the effects of reduced MEC proliferation observed in CM glands (Capuco et al., 1997). Rastani et al. (2003) also demonstrated that milk yields were unchanged in cows dry 28 d and 56 d. Gulay et al. (2003) examined the effects of a 30-d dry period, estrogen treatment at dry-off, and low-dose (143 mg/14 d compared to the FDA-approved dose 500 mg/14 d) administration of bST during the last 28 d of gestation and first 60 d postpartum on subsequent milk yields. Shortening the dry period and estrogen treatment did not alter milk yield (Table 1). bST treatment enhanced milk yield by 8%, but there was no interaction between dry period length and bST supplementation. An evaluation of a 30-d dry period in cows supplemented with bST (500 mg/14 d) according to label instructions (label = bST started at 57 to 70 DIM to end of lactation) resulted in subsequent milk yields in multiparous cows equal to 60-d dry multiparous cows but reduced milk yield in 30-d dry primiparous cows (Table 1) (Annen et al., 2004d).
The effect of continuous milking on subsequent milk yield and mammary cell turnover and ultrastructure has also been examined recently. Annen et al. (2004d) examined the effects of continuous milking with label (CMLST) and continuous (CMCST) bST supplementation during late gestation and early lactation (1 to 17 wk). Multiparous cows in CMLST and CMCST treatments had subsequent milk yields equal to controls (60-d dry), but primiparous cows in these treatments had 13 to 20% production losses (Table 1). Additional days of milk income during late gestation combined with the absence of reduced milk yield made CMLST and CMCST multiparous cows more profitable than controls during the first 17 weeks of the next lactation. Production losses in CMLST and CMCST primiparous cows negated income generated from milk produced during late gestation. Annen et al. (2004d) hypothesized that continuous milking impedes continued mammary development in primiparous cows, whereas multiparous cows no longer have a mammary growth requirement. This parity sensitivity to dry period length has also been detected in other commercial trials (Remond et al., 1997) and retrospective studies (Dias and Allaire, 1982). Remond et al. (1997) discussed data from a large commercial trial (Brittany Survey), which reported larger production losses in CM primiparous cows than multiparous cows. Dias and Allaire (1982) determined that as age at calving in the lactation before a shortened dry period increased from 24 to 83 months, the dry period requirement for optimal milk yield decreased from 65 to 23 d. As previously mentioned, data from retrospective studies may have biases and should be interpreted with caution.
Annen et al. (2004a) conducted a half-udder study to further investigate the effects of bST and continuous milking on MEC turnover and ultrastructure, as well as milk production in primiparous cows. MEC turnover was negatively impacted in CM halves. In tissue from 60-d dry (CTL) halves, MEC proliferation increased to d 8 prepartum, then decreased at the onset of copious secretion in early lactation (Figure 2), whereas MEC proliferation peaked at 20 prepartum in CM halves and declined thereafter. At d 8 prepartum, there was 50% less proliferation in CM tissue compared to CTL tissue (Figure 2).

The other aspect of MEC turnover, apoptosis, has not previously been measured in CM glands but is required to add merit to the hypothesis that CM glands carry more old cells into the subsequent lactation than CTL glands. Apoptotic indexes for MEC in CM and CTL glands were similar during late gestation (Figure 3). However, during elevated MEC apoptosis in early lactation, differences between the halves were observed. Apoptotic indexes were elevated through the first 7 d postpartum in CTL halves (Figure 3). In CM halves, MEC apoptosis was only elevated at 1 d postpartum and declined to prepartum levels thereafter (Figure 3). High levels of apoptosis are expected during the early dry period but have only recently been evaluated and reported in early lactation (Capuco et al., 2001; Hale et al., 2003; Sorensen and Sejrsen, 2003). It is believed that this increase in apoptosis is required for shedding of old or dormant MEC that were replaced with new MEC during late gestation or for removal of new MEC that did not fully differentiate into a secretory phenotype during lactogenesis (Sorensen et al., 2003). Removal of dormant and undifferentiated MEC may enable atrophy and increased secretory capacity of remaining MEC. The premature decline in apoptosis in CM tissue may be a mechanism by which total MEC numbers are maintained (Swanson, 1965; Capuco et al., 1997) despite reductions in proliferation during late gestation. This report of reduced MEC proliferation, accompanied by reductions in apoptosis, supports the hypothesis that MEC turnover is altered by CM and increases the proportion of old MEC that are carried into the subsequent lactation.

Additional support to the aforementioned hypothesis comes from ultrastructure analysis of CM and CTL tissue from late gestation and early lactation. Ultrastructural differences during late gestation were as expected. CM tissue contained lactating and resting alveoli at 20 d prepartum and lactating and immature (approaching a secretory phenotype) alveoli at 8 d prepartum. At 20 d prepartum, CTL tissue was composed of resting alveoli and by 8 d prepartum alveoli were in an immature secretory phenotype in response to lactogenic signals. During the first week postpartum, ultrastructure was similar in CM and CTL tissue. Tissue from both treatments was composed of lactating and immature alveoli. By 20 d postpartum, dramatic differences were evident between CM and CTL tissue. CTL tissue consisted of a homogeneous population of lactating alveoli (Figure 4), whereas CM tissue was composed of lactating, engorged (involuting; approaching a resting state), and resting alveoli (Figure 4). Large populations of engorged and resting alveoli at 20 d into the subsequent lactation when milk yield would still be increasing toward peak milk production were unexpected. This result is likely caused by more old cells in CM tissue prematurely shutting down to a resting state. This process normally should not occur until after peak milk yield and the start of the decline phase of the lactation cycle.
MEC apoptosis, proliferation, and ultrastructure were not affected by bST treatment. Prepartum half-udder milk yield in the CM halves was increased by 20% in bST-treated cows. Postpartum milk yield was not altered by bST treatment. Responses to bST during early lactation are not expected due to low or negative energy balance of the animal (Vicini et al., 1991; McGuire et al., 1992). Additionally, milk yield increases in response to a treatment during early lactation are difficult to detect because milk yield is already increasing during this phase of the lactation cycle.

Fitzgerald et al. (2004) evaluated the effects of both bST and IMF on recouping losses in milk yield in CM glands. The study utilized primiparous cows in a half-udder model. Cows were milked twice daily until parturition; after parturition, cows were milked four times daily (4x). Neither 4x milking nor bST alleviated production losses (30 to 40%) in CM udder halves. Similar to observations by Annen et al. (2004a), MEC proliferation and apoptotic indices and postpartum milk yield were not affected by bST, but prepartum half-udder yields were increased in bST-treated cows. Rastani et al. (2003) also evaluated the effects of omitting the dry period but without bST supplementation. Similar to previous reports on continuous milking (Swanson, 1965; Smith et al., 1967; Remond et al., 1992), the CM cows had a 14% reduction in daily milk yield in the following lactation when compared to controls (56 d dry).
The results from these continuous milking studies suggest a role for bST in improving synthetic capacity of old MEC in CM cows, especially multiparous cows, but bST supplementation does not alter MEC proliferation or apoptosis, nor does it reduce the negative effects of CM subsequent milk yield in primiparous cows. Studies evaluating mammary DNA content and gene expression in mammary tissue from CM, primiparous cows are required to understand the effects of modified dry periods on mammary development.
Finally, Annen et al. (2004b) conducted a two-part study that 1) compared MEC turnover in CM and involuting tissue and 2) evaluated the effects of CM and intramammary infusion of prostaglandin E2 (PGE) on early-lactation milk yield. The study used first- and second-lactation cows in a half-udder model. The first part of the experiment was designed to evaluate the changes in MEC turnover that occur when lactation is maintained in the CM half, but the 60-d dry (CTL) half is dried and undergoing involution. In CTL halves, apoptosis was increased at 72 h after dry-off compared to 7 d after dry-off, suggesting apoptosis and early stages of mammary involution were subsiding by d 7. Apoptosis in CM halves remained unchanged at these same sampling timepoints. MEC proliferation was equal in CM and CTL tissue at 72 h after dry-off of the CTL half. By d 7 after dry-off of the CTL half, MEC proliferation was three-fold greater in CTL tissue compared to CM tissue. In the second part of this experiment, PGE infusions (875 mg) were given at parturition and 72 h postpartum. PGE has been demonstrated as mammogenic in late pregnant heifers (Collier et al., 2002) and mitogenic to MEC (McGrath et al., 1990; Lacasse et al., 1996). It was hypothesized that intramammary infusion of PGE would stimulate MEC proliferation in CM glands and compensate for reductions in proliferation observed throughout late gestation in CM glands. The use of PGE to promote mammary growth was unsuccessful. Neither MEC proliferation nor apoptosis was altered by PGE treatment. Additionally, PGE did not alter milk yield. Milk yield was reduced in CM halves, and second-lactation cows were less affected by CM than first-lactation cows. Milk yield was reduced by 50% in first-lactation cows and 32% in second-lactation cows. Results suggest that levels of mammary PGE2 content during parturition are not limiting to MEC proliferation or milk yield in CM glands.
In summary of recent research, primiparous cows continue to require a 60-d dry period despite production and technological advances in the dairy industry. Multiparous cows are good candidates for a shortened dry periods (30 to 40 d) and potentially for continuous milking if combined with bST supplementation. The use of galactopoietics (bST and PGE) and IMF to alleviate production losses in were unsuccessful in primiparous cows. The animals in these shortened dry period or continuous milking studies (Bachman, 2002; Gulay et al., 2003; Rastani et al., 2003; Annen et al., 2004a, b, d; Fitzgerald et al., 2004) had milk yields representative of today’s dairy cow (Table 1).
Effects of Modified Dry Periods on the Transition Period
As previously discussed, the last 60 d of gestation essentially has two transition periods, both of which are associated with increased health risks. The first transition period occurs during the early dry period when the mammary gland undergoes involution. Cessation of milking at dry-off results in increased risk of mastitis due to udder engorgement preventing teat sealing and the absence of bacterial flushing which normally occurs after milk removal (Smith et al., 1985). Further, udder engorgement during the first few days of the dry period is a discomfort to the animal. These effects are exacerbated by high yields at the time of dry-off. The diet change at dry-off to a less nutrient-dense ration is required to reduce substrate availability for milk production and minimize udder distention, but this change also poses as a stressor to an animal undergoing such dramatic physiological changes.
The other transition period occurs from three weeks prepartum to three weeks postpartum (Grummer, 1995). Parturition and the onset of lactation during a period of declining DMI results in increased risk of metabolic disease (i.e., ketosis, milk fever, retained placenta, and displaced abomasums) (Goff and Horst, 1997). Similar to the dry-off transition period, the periparturient transition period is complicated by a diet change at 21 d prepartum and again at parturition. DMI declines by 30 to 35% during the last three weeks of gestation (Grummer, 1995). Collier et al. (2004) reviewed DMI from numerous studies and a range of prepartum diets and reported an average DMI of 10 to 12 kg/d during the last three to four weeks of gestation. Minimizing the decline in DMI would prove beneficial in reducing health risks in early lactation cows and improving milk yield.
Reduced DMI during the final weeks of gestation occurs as nutrient demands for conceptus growth and the onset of lactation increase, creating a state of negative energy and possibly protein balance by the last week of gestation and during early lactation (Bell, 1995; Grummer, 1995). Negative energy and protein balance result in mobilization of body reserves to meet nutrient demands for pregnancy and lactation and predispose the animal to metabolic diseases (Goff and Horst, 1997). Improvements in DMI during this period would improve energy/protein balance, thus reducing health risks. As mentioned in a previous section, removal of both late-gestation transition periods would keep the cow metabolically and physiologically adapted to lactation and associated diets which may improve DMI and energy/protein balance during the final weeks of gestation and early lactation.
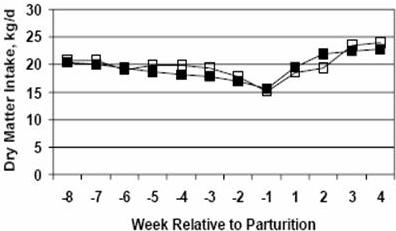
Continuous milking or 30-d dry periods have been suggested to improve energy balance (Lotan and Alder, 1976; Remond et al., 1992). Improved body weight, decreased plasma non-esterified fatty acid levels, increased blood glucose levels, and enhanced milk protein synthesis are all indicators of improved energetic status and have been demonstrated in cows given modified dry periods (Lotan and Alder, 1976; Remond et al., 1992). Other studies on modified dry periods have reported improved DMI (Gulay et al., 2003; Rastani et al., 2003). Cows dry 30 d tended to have increased DMI and lost less body condition than 60-d dry cows (Gulay et al., 2003). Data of Rastani et al. (2003) demonstrated 21% greater DMI in 28-d dry cows, and 30% greater DMI in CM cows compared to 56-d dry cows. DMI was also measured in the half-udder studies conducted by Annen et al. (2004a,b) and Fitzgerald et al. (2004). As long as the CM half was milking, cows were fed a high-energy, lactating diet. Cows that spontaneously dried the CM half before parturition were switched to a close-up, dry-cow diet (moderate energy) until parturition. This elimination or reduction in diet changes resulted in dramatic improvements in DMI. In the study by Annen et al. (2004a), DMI decreased by 17% over the last three weeks of gestation (Figure 5). Given the typical decrease of 30 to 35% during the last three weeks of gestation, the nutritional management protocols employed dramatically improved on this DMI decline. Further, DMI was in excess of 17 kg/d during the last three weeks of gestation and above 20 kg/d within three weeks postpartum. DMI was not altered by bST treatment. Similar results were obtained by Fitzgerald et al. (2004). Results reported by Annen et al. (2004b) demonstrated even greater improvements in DMI during late gestation and early lactation as a result of minimizing diet changes. DMI was 22 kg/d at three weeks prepartum and only declined to 19.1 kg/d on the day of parturition (Figure 6). By week 2 postpartum DMI exceeded 25 kg/d (Figure 6). Because both CM and PGE treatments were half-udder treatments, their effects on whole-animal DMI could not be tested. The use of both first- and second-lactation cows in this study contributed to improvements over results reported by Annen et al. (2004a) and Fitzgerald et al. (2004). Second-lactation cows had higher DMI throughout the study than first-lactation cows.
Conclusion
In conclusion of current research, there have been consistent reports of no production losses following a 30-d dry period with or without bST-supplementation (Bachman, 2002; Gulay et al., 2003; Rastani et al., 2003; Annen et al., 2004d). This may be a viable management practice in multiparous cows. Results from continuous milking studies have been more variable than observed with short dry periods. High-producing, CM cows not supplemented with bST produce less milk in the subsequent lactation than traditionally managed cows (Rastani et al., 2003). However, bST-supplemented multiparous cows produced milk yields equal to 60-d dry cows (Annen et al., 2004d). Modified dry periods in primiparous cows have resulted in consistent reports of reduced milk yield in the subsequent lactation (Annen et al., 2004a,b,d; Fitzgerald et al., 2004). This parity sensitivity to dry period length is likely the result of continued mammary development between the first and second lactations. In addition to promising production results in multiparous cows given a modified dry period, there are also promising results for improving DMI and energy/protein balance during the last three weeks of gestation and early lactation.

References
Annen, E.L., A.V. Capuco, P.C. Gentry, L.H. Baumgard, and R.J. Collier. 2003. Late gestation and advanced lactation at cessation of milking do not delay mammary epithelial apoptosis in dairy cattle. J. Dairy Sci. 86 (suppl. 1):117.
Annen, E.L., A.C. Fitzgerald, P.C. Gentry, and R.J. Collier. 2004a. Effects of continuous milking and bST supplementation on subsequent milk yield and composition and mammary epithelial cell proliferation. J. Dairy Sci. 87(suppl. 1):132.
Annen, E.L., C.M. Steining, A.C. Fitzgerald, M.E. Dwyer, B.A. Crooker and R.J. Collier. 2004b. Effects of continuous milking and intramammary infusion of prostaglandin E2 on subsequent milk yield and composition. J. Dairy Sci. 87(suppl. 1):132.
Annen, E.L., R.J. Collier, M.A. McGuire, and J.L. Vicini. 2004c. Effect of dry period length on milk yield and mammary epithelial cells. J. Dairy Sci. 87(E Suppl.):E66-E76.
Annen, E.L., R.J. Collier, M.A. McGuire, J.L. Vicini, J.M. Ballam, and M.J. Lormore. 2004d. Effect of modified dry period lengths and somatotropin protocols on milk yield and composition of primiparous and multiparous cows. J. Dairy Sci. 87:3746-3761.
Bachman, K.C. 2002. Milk production of dairy cows treated with estrogen at the onset of a short dry period. J. Dairy Sci. 85:797-803.
Bachman, K.C. and M.L. Schairer. 2003. Invited review: Bovine studies on optimal lengths of dry periods. J. Dairy Sci. 86:3027-3037.
Bachman, K.C., D.H. Wilfond, H.H. Head, C.J. Wilcox, and M. Singh. 1992. Milk yields and hormone concentrations of Holstein cows in response to Sometribove (Somatotropin) treatment during the dry period. J. Dairy Sci. 75:1883-1890.
Bauman D.E., R.W. Everett, W.H. Weiland, and R.J. Collier. 1999. Production responses to bovine somatotropin in northeast dairy herds. J. Dairy Sci. 82:2564-2573.
Bauman, D.E. and R.G. Vernon. 1993. Effects of exogenous bovine somatotropin on lactation. Annu. Rev. Nutr. 13:437-461.
Bell, A.W. 1995. Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. J. Anim. Sci. 73:2804-2819.
Capuco, A.V. and R.M. Akers. 1999. Mammary involution in dairy animals. J. Mam. Gland Bio. Neoplasia 4:137-144.
Capuco, A.V., D.L. Wood, R. Baldwin, K. McLeod, and M.J. Paape. 2001. Mammary cell number, proliferation, and apoptosis during a bovine lactation: Relation to milk production and effect of bST. J. Dairy Sci. 84:2177-2187.
Capuco, A.V., E.L. Annen, A.C. Fitzgerald, S.E. Ellis. In Press. Mammary cell turnover: Relevance to lactation persistency and dry period management. In Proc.: 2004 International Symposium on Ruminant Physiology. Copenhagen, Denmark. Wageningen Academic Publishers. August 30-September 4, 2004.
Capuco, A.V., R.M. Akers, and J.J Smith. 1997. Mammary growth in Holstein cows during the dry period: Quantification of nucleic acids and histology. J. Dairy Sci. 80:477-487.
Collier, R.J., E.L. Annen, and A.C. Fitzgerald. 2004. Prospects for zero days dry. The Veterinary Clinics of North America: Food Animal Practice, Transition Cow Management Issue. Vet. Clin. North Am. Food Anim. Pract. 20:687-701.
Collier, R.J., J.C. Byatt, M.F. McGrath, P.J. Eppard, J.L. Vicini, and C. Steining. 2002. Effect of growth factors and hormones on mammogenesis and lactogenesis. J. Dairy Sci. 85 (suppl. 1):53 (Abstr).
Coppock, C.E., R.W. Everett, R.P. Natzke, and H.R. Ainslie. 1974. Effect of dry period length on Holstein milk production and selected disorders at parturition. J. Dairy Sci. 57:712-717.
Dias, F.M., and F.R. Allaire. 1982. Dry period to maximize milk production over two consecutive lactations. J. Dairy Sci. 65:136-145.
Dix Arnold, P.T., and R.B. Becker. 1936. Influence of preceding dry period and of mineral supplementation on lactation. J. Dairy Sci. 19:257-266.
Fitzgerald, A.C., E.L. Annen, P.C. Gentry, L.H. Baumgard, and R.J. Collier. 2004. Effects of continuous milking, bST, and early-lactation increased milking frequency on mammary cell proliferation, milk yield, and composition in primiparous cows. J. Dairy Sci. 87(suppl. 1):425.
Fowler, P.A., C.H. Knight, and M.A. Foster. 1991. Omitting the dry period between lactations does not reduce subsequent milk production in goats. J. Dairy Res. 58:13-19.
Furth, P.A. 1999. Mammary gland involution and apoptosis of mammary epithelial cells. J. Mam. Gland Bio. Neoplasia 4:123-138.
Goff, J.P. and R.L. Horst. 1997. Physiological changes at parturition and their relationship to metabolic disorders. J. Dairy Sci. 80:1260-1268.
Grummer, R.R. 1995. Impact of changes in organic nutrient metabolism on feeding the transition dairy cow. J. Anim. Sci. 73:2820-2833.
Grummer, R.R. and R.R. Rastani. 2004. Why reevaluate dry period length? J. Dairy Sci. 2004 87(E suppl):E77-E85.
Gulay, M.S., M.J. Hayen, K.C. Bachman, T. Belloso, M. Liboni, and H.H. Head. 2003. Milk production and feed intake of Holstein cows given short (30-d) or normal (60-d) dry periods. J. Dairy Sci. 86:2030-2038.
Hale, S.A., A.V. Capuco, and R.A. Erdman. 2003. Milk yield and mammary growth effects due to increased milking frequency during early lactation. J. Dairy Sci. 86:2061-2071.
Holst, B.D., W.L. Hurley, and D.R. Nelson. 1987. Involution of the bovine mammary gland: Histological and ultrastructural changes. J. Dairy Sci. 70:935-944.
Hurley, W.L. 1989. Mammary gland function during involution. J. Dairy Sci. 72:1637-1646.
Knight, C.H. 1998. Extended lactation. Hannah Research Institute Yearbook 1998. pp. 30-39.
Lacasse, P., E. Block, J. Turner, T. Woodward, Y. Couture, and D. Petitclerc. 1996. Evolution of insulin-like growth factor-I, prostaglandin E2, and mitogenic activity of bovine mammary primary lymph during the dry period and lactogenesis. J. Dairy Sci. 79:1746-1753.
Lotan, E. and J.H. Alder. 1976. Observations on the effect of shortening the dry period on milk yield, body weight, and circulating glucose and FFA levels in dairy cows. Tijdschr. Diergeneesk. 101:77-82.
McGrath, M.F., L.A. Kaempfe, C.A. Sweeny, and R.J. Collier. 1990. The production and function of prostaglandin E2 by bovine mammary cells grown in collagen gel culture. J. Dairy Sci. 73(suppl. 1):214(Abstr).
McGuire, M.A., D.E. Bauman, D.A. Dwyer, and W.S. Cohick. 1995. Nutritional modulation of the somatotropin/insulin-like growth factor system: Response to feed deprivation in lactating cows. J. Nutr. 125:493-502.
Paape, M.J. and H.A. Tucker. 1969. Influence of length of dry period on subsequent lactation in the rat. J. Dairy Sci. 52:518-522.
Pitkow, H.S., R.P. Reece, and G.L. Waszilycsak. 1972. The integrity of mammary alveolar cells in two consecutive lactations. Proc. Soc. Exp. Biol. Med. 139:845-850.
Rastani, R.R., R.R Grummer, S.J. Bertics, A. Gümen, M.C. Wiltbank, D.G. Mashek, and M.C. Rich. 2003. Effects of varying dry period length and prepartum diet on metabolic profiles and lactation of periparturient dairy cattle. J Dairy Sci. 86(suppl. 1):154(Abstr).
Remond, B., A. Ollier, and G. Miranda. 1992. Milking cows in late pregnancy: milk production during this period and during the succeeding lactation. J. Dairy Res. 59:233-241.
Remond, B., J. Kerouanton, and V. Brocard. 1997. Effets de la réduction de la durée de la période séche ou de son omission sur les performances des vaches laitiéras. INRA Prod. Anim. 10:301-333.
Sejrsen, K., S. Purup, M. Vestergaard, and M.T. Sorensen. 2003. Fodringens betydning for mælkekirtlernes vækst og udvikling. In: Kvægets ernæring og fysiologi (vol. 1). DJF Report number 53. pp. 463-487.
Smith, A., J.V. Wheelock, and F.H. Dodd. 1967. Effect of milking throughout pregnancy on milk secretion in the succeeding lactation. J. Dairy Res. 34:145-150
Sorensen, J.T. and C. Enevoldsen. 1991. Effect of dry period length on milk production in subsequent lactation. J. Dairy Sci. 74:1277-1283.
Smith, K.L., D.A. Todhunter, and P.S. Schoenberger. 1985. Environmental pathogens and intramammary infection during the dry period. J. Dairy Sci. 68:402-417.
Sorensen, J.T. and C. Enevoldsen. 1991. Effect of dry period length on milk production in subsequent lactation. J. Dairy Sci. 74:1277-1283.
Sorensen, M.T. and K. Sejrsen. 2003. Mammary cell proliferation, apoptosis, and enzymatic activity during pregnancy and lactation in dairy cows. In Proc.: 54th Annual Meeting of the European Association for Animal Production. Rome, Italy. Commission on Animal Physiology, Session Ph 4.1 Free Communications.
Stelwagen, K., D.G. Grieve, J.S. Walton, J.L. Ball, and B.W. McBride. 1993. Effect of prepartum bovine somatotropin in primigravid ewes on mammogenesis, milk production, and hormone concentrations. J. Dairy Sci. 76:992-1001.
Swanson, E. W. 1965. Comparing continuous milking with 60-day dry periods in successive lactations. J. Dairy Sci. 48:1205-1209.
Swanson, E. W., F. E. Pardue, and D. B. Longmire. 1967. Effect of gestation and dry period on deoxyribonucleic acid and alveolar characteristics of bovine mammary glands. J. Dairy Sci. 50:1288-1292.
United States Department of Agriculture, Animal and Plant Health Inspection Service, Veterinary Services. 2002. Dairy 2002. Part 1: Reference of Dairy Health and Management in the United States, 2002. USDA, Anim. Plant Health Inspection Serv., Vet. Serv., Ctr. Epidemiol. Anim. Health, Natl. Anim. Health Monitoring Syst., Ft. Collins, CO. Info. Sheet No. N377.1202.
Vicini, J.L., F.C. Buonomo, J.J. Veenhuizen, M.A. Miller, D.R. Clemmons, and R.J. Collier. 1991. Nutrient balance and stage of lactation affect responses of insulin, insulin-like growth factors I and II, and insulin-like growth factor-binding protein 2 to somatotropin administration in dairy cows. J. Nutr. 121:1656-1664.
| Reference | Model | Dry period length | Results | Peak Milk Yield in Controls |
| Previous Research | ||||
| Swanson, 1965 | Identical twins | CM |
|
18-20 kg/d |
| Smith et al., 1967 | Half-udder | CM |
|
120-25 kg/d |
| Coppock et al., 1974 | Between animal | 20, 30, 40, and 50 d |
|
6726 kg 305-d Mature Equivalent Milk Yield |
| Lotan and Alder, 1976 | Between animal | 30 d |
|
Less than 34 kg/d |
| Sorensen and Enevoldsen, 1991 | Between animal | 4 wk |
|
25 kg/d average milk yield through 84 DIM |
| Remond et al., 1992 | Between animal (Primiparous cows) | CM |
|
Less than 30 kg/d |
| Current Research | ||||
| Bachman, 2002 | Between animal | 30 d |
|
46 kg/d |
| Gulay et al., 2003 | Between animal | 30 d |
|
44 kg/d |
| Rastani et al., 2003 | Between animal | 28 d and CM |
|
42 kg/d |
| Annen et al., 2004d | Between animal | 30 d and CM |
|
Greater than 45 kg/d (primiparous) Greater than 50 kg/d (multiparous) |
| Annen et al., 2004a | Half-udder (Primiparous cows) | CM |
|
1Greater than 45 kg/d |
| Annen et al., 2004b | Half-udder (first- and second-lactation cows) | CM |
|
1Greater than 49 kg/d |
| Fitzgerald et al., 2004 | Half-udder (Primiparous cows) | CM |
|
1Greater than 45 kg/d |
|
1Peak milk yield calculated by doubling half-udder yields in 60-d dry (control) udder halves 230DD = 30-d dry period, label bST, CMLST = no dry period, label bST and CMCST = no dry, continuous bST supplementation 3MEC = mammary epithelial cell |
||||
Author Information
Ehrin L. Annen
Robert J. Collier
University of Arizona
2005 Proc. Southwest Nutr. Conf.:209-224.
