Contents |
Introduction
Cottonseed is a product of the cotton-fiber industry that is extensively used as an energy and protein source in dairy cattle diets. It provides a unique blend of protein, energy, and fiber compared with other feedstuffs. Cottonseed is fed to dairy cows at 10 to 15% of the total diet dry matter (DM). Diets containing more than 15% cottonseed generally are high in fat and may contain undesirable concentrations of gossypol.
Two major types of cottonseed are available in the United States, Upland and Pima. Upland is a high-lint type of cottonseed that is generally fed whole, while Pima is a delinted type of cottonseed that is often fed either cracked or ground to improve nutrient utilization. The nutrient composition of these two types of cottonseed differs, and Pima is often considered nutritionally superior because of its higher fat and protein contents. In addition, Pima cottonseed contains more gossypol than whole linted Upland cottonseed, and more of its gossypol is represented by the minus (-) isomer.
Gossypol is a yellow, polyphenolic compound found primarily in the pigment glands of the cotton plant, and it exists in both the free and bound forms. In the intact whole seed, gossypol is mostly found as free gossypol (FG). However, when cottonseed is processed, gossypol binds to proteins, possibly to the epsilon-amino group of lysine. Two distinct stereoisomer forms of gossypol occur in cottonseed, the plus (+) and the minus (-) isomers. The (-) isomer has greater biological activity and is retained in the body for a longer period of time.
Ruminants with a well-developed rumen microbial population are able to detoxify gossypol by converting free gossypol to bound gossypol within the rumen, thereby impeding its absorption into the blood. However, it is possible that feeding excessive amounts of gossypol in the free form may exceed this protective mechanism and impair animal performance.
Please check this link first if you are interested in organic or specialty dairy production.
Nutrient Characterization and Gossypol Content of Cottonseeds
Whole cottonseed provides a unique blend of energy, protein, and fiber in the diet of dairy cattle. It is usually one of the most competitive sources of fat and protein supplied to dairy cattle and commonly used in lactating rations throughout the United States. Most of the protein and fat in cottonseed is contained within the meats, and the seed coat and lint provide most of the neutral detergent fiber (NDF).
The protein in the cottonseed kernel consists of storage proteins that are readily available in the rumen. Depending upon rumen retention time, rumen protein degradability of cottonseed is generally high, which reflects the increased rumen ammonia N often observed after in vitro incubation. Based on in vitro incubation of whole cottonseed, rumen protein degradability was estimated to be approximately 70% when rumen outflow rate is 0.06/hr (Arieli, 1998).
Fat in cottonseed is mostly in the form of oil, and unsaturated fatty acids are the predominant fatty acids. The polyunsaturated fatty acid linoleic acid is the main fatty acid in cottonseed oil, and it represents up to 50% of the total fat. Smaller quantities of oleic and palmitic acids are observed in cottonseed oil. The triglycerides in cottonseed oil are readily available in the rumen. They are hydrolyzed, and unsaturated free fatty acids are biohydrogenated by rumen microorganisms.
The type of cotton affects the nutrient content in the seed. Whole linted cottonseed contains more fiber and less protein and fat than delinted cottonseed. A survey of 31 oil mills across the United States showed a mean concentration of 22.5% crude protein (CP), 38.8% acid detergent fiber (ADF), 47.2% NDF, and 17.8% ether extract in the DM (Calhoun et al., 1995). Recent data from whole linted Upland cottonseed and Pima cottonseed in California showed that cracked Pima cottonseed contained more fat and CP and less NDF than whole linted Upland cottonseed (Table 1).
| Cottonseed | ||
|---|---|---|
| Whole Linted Upland | Cracked Pima | |
| Samples, number | 18 | 18 |
| DM, % | 92.2 | 92.4 |
| NEL1, Mcal/lb | 0.90 | 0.92 |
| —————————————————- DM basis —————————————————– | ||
| OM, % | 95.83 | 95.20 |
| CP, % | 26.03 | 30.47 |
| Fat, % | 23.03 | 25.37 |
| NDF, % | 52.53 | 38.57 |
| ADF, % | 40.03 | 31.23 |
| Ash, % | 4.17 | 4.80 |
| Ca, % | 0.18 | 0.21 |
| P, % | 0.79 | 0.89 |
| Mg, % | 0.41 | 0.45 |
| K, % | 1.15 | 1.28 |
| Na, % | 0.03 | 0.01 |
| Fe, mg/kg | 76.67 | 63.67 |
| Zn, mg/kg | 38.00 | 41.67 |
| Cu, mg/kg | 7.40 | 10.17 |
| Mn, mg/kg | 15.47 | 14.83 |
| 1NEL = Net energy for lactation; calculated from nutrient composition according to NRC (2001). | ||
The variety of cotton also affects the nutrient content of the seed. Robinson et al. (2001) observed that the protein content of Pima cottonseed changed with variety, but fat and fiber were similar. Nevertheless, the values of protein and fat reported by Robinson et al. (2001) were generally higher than those observed for whole linted Upland cottonseed. Others (Coppock et al., 1985; NRC, 2001; Prieto et al., 2003; Sullivan et al., 1993a) also reported that linted cottonseed contained less CP and fat and more NDF than delinted cottonseed.
Concentration of gossypol in cottonseed is variable and usually higher for Pima than whole linted Upland cottonseed (Table 2). On average, total gossypol (TG) content of cracked Pima and whole linted Upland cottonseed were, respectively, 1.03% (± 0.01) and 0.69% (± 0.01) on an as is basis, and 1.12% (± 0.01) and 0.74% (± 0.01) of the total DM. More than 95% of the TG was FG in whole linted Upland cottonseed and cracked Pima cottonseed. Cracked Pima cottonseed also has a higher proportion of the TG as the (-) isomer than whole linted Upland cottonseed. Robinson et al. (2001) reported similar concentrations of TG and FG in four Pima cottonseed varieties harvested in California. They also reported that the (-) gossypol isomer represented approximately 52% of the TG in Pima cottonseed, which is similar to the values reported in Table 2 for cracked Pima but higher than those observed for whole Upland cottonseed. As a consequence of processing and low lint content, cracked Pima cottonseed has a smaller mean particle size and a higher bulk density than whole Upland cottonseed.
| Cottonseed | |||
|---|---|---|---|
| Whole Linted Upland | Cracked Pima | ||
| Samples, number | 18 | 18 | |
| Gossypol, as is | Total, % | 0.69 | 1.03 |
| Free, % | 0.66 | 0.98 | |
| Gossypol, DM basis | Total, % | 0.74 | 1.12 |
| Free, % | 0.72 | 1.06 | |
| Gossypol isomers | (-), % DM | 0.31 | 0.56 |
| (-), % total gossypol | 42.4 | 52.4 | |
| (+), % DM | 0.41 | 0.51 | |
| (+), % total gossypol | 57.8 | 47.8 | |
| Density, kg/L | 0.24 | 0.36 | |
| Mean particle size, mm | 8.17 | 3.03 | |
Plasma Gossypol Concentrations
Plasma gossypol concentrations directly reflect gossypol absorption in the gut and metabolism by the liver. When cottonseed is fed, gossypol in the free form that escapes detoxification within the forestomach is available for absorption in the small intestine. It is assumed that binding of gossypol to amino acids of dietary and bacterial origin reduces gossypol availability. When cottonseed escapes ruminal digestion, plasma gossypol concentrations increase dramatically.
Mena et al. (2001) fed lactating Holstein cows varying amounts of gossypol from whole linted Upland cottonseed or cottonseed meal. Gossypol in cottonseed is mostly in the free form, and gossypol in cottonseed meal is mostly in the bound form. Plasma gossypol concentrations were directly related to intake of FG (Figure 1).

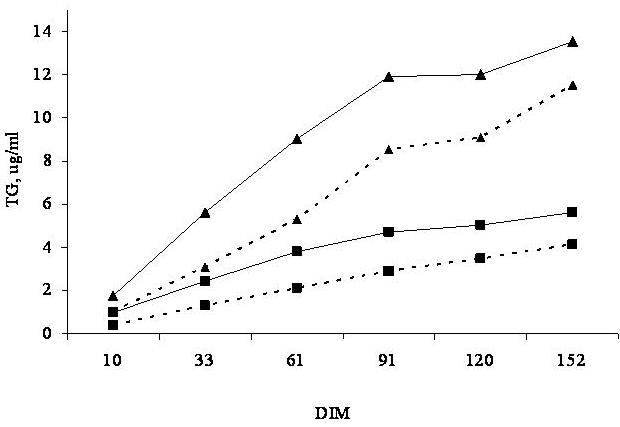
A field trial was conducted in California to determine the effects of type of cottonseed and gossypol intake on plasma gossypol concentrations of lactating cows during the first 183 days in lactation (Santos et al., 2002). Eight hundred and thirteen cows from three commercial dairy farms were fed either 6 lb/day of whole linted Upland cottonseed or a blend of 2 lb/day of whole linted Upland cottonseed with 4 lb/day of cracked Pima cottonseed. Blood samples were collected monthly and analyzed for plasma gossypol concentration during the first 150 days in milk (DIM). Although FG intake increased only 32% when cracked Pima replaced a portion of the whole linted Upland cottonseed, mean plasma TG during the first 150 days in lactation more than doubled in cows fed the blend of Upland and cracked Pima compared with those fed only whole Upland cottonseed (7.76 vs. 3.07 µg/ml; P < 0.001). Furthermore, the increase in plasma TG with time of feeding was higher for multiparous cows fed the blend of Upland and cracked Pima than for those fed whole Upland only (Figure 2).
Previously, Mena et al. (2001) observed that PG concentrations plateaued after 28 to 35 days of feeding cottonseed in mid-lactation cows (Figure 3). The data from Santos et al. (2002) suggest that cows fed diets with 10% cottonseed and consuming 17 to 24 g/d of FG still have not reached a plateau PG at 150 DIM. This is probably related to the level of DM intake of those cows. When DM intake is stable, PG concentrations plateau after four to five weeks.
The increases in plasma TG when cows are fed a blend of whole Upland and cracked Pima cottonseed cannot be explained based solely upon the gossypol content of the diet and the FG intake. In a previous study with Holstein steers, Santos et al. (2005) observed that processing of whole cottonseed by cracking increased plasma concentrations of TG, although TG intake measured as g/d or as mg/kg of live weight per day did not differ. More recently, Prieto et al. (2003) observed that when cracked Pima replaced all the whole Upland cottonseed in the diet, TG intake increased 57% and 41% for primiparous and multiparous cows, respectively, while plasma TG concentrations more than doubled in both groups of cows compared with those fed whole linted Upland cottonseed.

The major site for gossypol detoxification is the reticulum-rumen (Calhoun et al., 1995). When lint on whole cottonseed is removed, passage rate of cottonseed through the digestive tract was increased (Coppock et al., 1985), which suggests that rumen retention time was reduced. When whole linted cottonseed was compared with whole Pima and ground Pima in the diet of finishing steers, feeding whole linted cottonseed increased ruminal digestion of organic matter and ADF (Zinn, 1995), thereby supporting the hypothesis that rumen retention time of the cottonseed might be decreased by lack of lint and by seed processing through cracking or grinding. We observed that cracked Pima cottonseed had a smaller mean particle size and a greater bulk density than whole linted Upland cottonseed (Table 2). Such changes in the physical characteristics of feedstuffs are usually associated with an increased ruminal passage rate. Mena et al. (2001) proposed that gossypol detoxification within the rumen might be reduced when rumen retention time of gossypol containing feeds is reduced.
Therefore, it might be expected that feeding cracked Pima cottonseed will increase PG concentrations due to the greater gossypol content in Pima compared to whole linted Upland cottonseed, as well as due to the lack of lint and the cracking of cottonseed, which seems to increase gossypol availability for absorption, probably by reducing its detoxification within the rumen.
Processing of cottonseed by pelleting and addition of iron sulfate to the diet seem to decrease gossypol availability and PG concentrations in growing steers and lactating dairy cows. However, when iron sulfate was added to diets of lactating cows containing cracked Pima cottonseed, milk production decreased (Ed DePeters, University of California-Davis, personal communication).
Feed Intake and Lactation Performance
Feeding cottonseed in dairy rations does not alter DM intake of lactating cows. Coppock et al. (1987) concluded that whole cottonseed can be added up to 25% of the diet DM with no effects on intake. When the fiber content of the diet is maintained constant, adding whole cottonseed to the diet of lactating cows at 10 to 20% usually has no effect on DM intake. This is extremely important since nutrient intake is the driving force for yields of milk and milk components.
Mena et al. (2001) studied the effects of feeding diets with different free and total gossypol contents to mid-lactation cows. Diet TG concentrations ranged from 0 up to 1,900 mg/kg of DM, and no effects were observed for total and free gossypol contents of the ration on intake of DM. Harrison et al. (1995) studied the effects of feeding diets with 12% whole linted cottonseed on DM intake of cows in two herds. In one herd in which diets were kept with similar energy content, adding whole cottonseed increased DM intake. In the second herd, adding whole cottonseed increased the energy content of the diet and decreased DM intake. Prieto et al. (2003) fed graded amounts of Pima cottonseed to primiparous and multiparous cows. No effects of either type of cottonseed or gossypol content of the diet were observed for intakes of DM. Santos et al. (2002) also observed no effect of either type of cottonseed or dietary gossypol concentration on intake of nutrients.
A comprehensive review of the effects of whole cottonseed on lactation of dairy cows showed that including 10 to 30% of the diet as whole cottonseed increased milk fat content in four out of 13 trials (Coppock et al., 1987). In general, adding whole cottonseed to the diet of lactating cows has minimal effect on milk fat content. Some have suggested that addition of whole cottonseed to diets high in corn silage can be detrimental to milk fat content (Smith et al., 1993). This negative effect may be related to the fat content of the diet, as well as the availability of physically effective fiber in high corn silage diets. Neither type of cottonseed (Pima vs. Upland) nor gossypol intake had any effect on milk fat content (Prieto et al., 2003; Santos et al., 2002; Sullivan et al., 1993b).
When cottonseed is added to the diet of lactating cows and the fat content of the ration increases, usually a milk protein content depression is observed (Coppock et al., 1987). However, yields of milk protein and fat either do not change or increase with addition of cottonseed to the ration. The effect of whole cottonseed on milk protein is usually negative when corn silage is the main forage.
The association between gossypol intake and lactation performance in dairy cows is an important issue for dairy producers. It is clear that gossypol can potentially be toxic to animals, mainly to those devoid of pre-gastric fermentation of feeds. Young ruminants and monogastric animals are highly susceptible to the negative effects of gossypol on health and fertility. However, ruminant animals can tolerate high amounts of gossypol in the diet with no clear adverse effects on lactation performance. Mena et al. (2001) reported that cows consuming more FG in the diet had higher concentrations of PG and produced more milk. Prieto et al. (2003) observed no negative effect of increasing dietary gossypol by adding cracked Pima cottonseed to the diet on yields of milk and milk components.
Santos et al. (2002) observed that cows producing more actual milk, 3.5% fat-corrected milk, milk fat, and milk true protein also had higher PG concentrations during the first 183 DIM (Table 3). Plasma TG concentration is directly related to FG intake, and it is likely that the increases in yields of milk and milk components in cows with plasma TG concentrations above the mean were in response to higher DM intakes and consequently higher FG intakes. This suggests that PG concentrations may not be a good predictor of impacts of cottonseed on production performance.
| Plasma TG | Effect: P <2 | |||
|---|---|---|---|---|
| Above Mean | Below Mean | Gossypol | TRT x Gossypol | |
| Milk, lb/day | 93.1 | 84.3 | 0.01 | 0.90 |
| 3.5% FCM, lb/day | 95.7 | 84.7 | 0.001 | 0.54 |
| Milk fat % lb/day |
3.65 3.41 |
3.54 2.97 |
0.32 0.001 |
0.44 0.40 |
| Milk true protein % lb/day |
2.90 2.70 |
2.92 2.46 |
0.54 0.007 |
0.61 0.94 |
| 1Taken from (Santos et al., 2002); FCM = fat corrected milk. 2 TRT x gossypol = interaction between treatment and plasma total gossypol. | ||||
These data neither suggest that gossypol cannot be detrimental to lactating cow performance nor indicate that gossypol is not toxic to dairy cows. However, when diets contain up to 20% whole linted cottonseed or up to 950 mg/kg of FG from a blend of 1:2 whole linted Upland cottonseed and cracked Pima cottonseed, dietary gossypol seems not to affect yields of milk or milk components. This suggests that cottonseed and known amounts of gossypol can be safely fed to lactating dairy cows with no adverse effects on lactation.
Reproduction and Health
Gossypol was first discovered by Chinese scientists after noticing that no birth of a child happened for more than a decade in a village where people cooked food with cottonseed oil. Since then, numerous reports in the literature have confirmed the anti-fertility effect of gossypol in mammals. Gossypol disrupts cell membrane metabolism and can cause rupture of red blood cells. In fact, erythrocyte fragility has been one of the indicators of gossypol toxicosis.
Risco et al. (1992) were one of the first to show that gossypol can be toxic and even kill growing cattle. They fed rations to bull calves with 200, 400, or 800 mg/kg of FG for 120 days. The diets with 400 and 800 mg/kg of FG were considered to be toxic and could potentially cause the death of growing ruminants. Baby calves have little ability to detoxify gossypol, and toxicity can be easily induced by feeding cotton products.
The negative effects of gossypol on fertility of ruminants are clear in males. Studies at the University of Florida and at Kansas State University have been shown that as little as 8 g/d of FG fed to young bulls reduced sperm quality and sexual activity (Chenoweth et al., 2000; Velzaquez-Pereira et al., 1998).
Little information is available on the effects of cottonseed or gossypol on fertility of dairy cows. The female ruminant seems to be relatively insensitive to the antifertility effect of gossypol, but in vitro data indicate some inhibition of embryonic development and ovarian steroidogenesis (Randel et al., 1992). However, a recent study from the University of Florida (Brocas et al., 1997) observed a distinct effect of gossypol on gametes and embryos of cattle. Feeding cottonseed meal to dairy cows did not affect the number of oocytes collected per cow, cleavage rate after in vitro maturation and fertilization, or the proportion of oocytes or embryos that developed to blastocysts. When oocytes were exposed in vitro to physiological levels of gossypol (similar to those observed in plasma of cows fed cotton products), cleavage rate or subsequent development was not affected. In contrast, addition of a higher gossypol concentration to the media reduced embryo cleavage rate. Based on these data, the authors concluded that developing embryos are sensitive to high gossypol concentrations and diets that increase plasma gossypol concentration may impact fertility of cows.
Three experiments were conducted to determine the effects of three concentrations of FG in the diet of Holstein dairy heifers on reproductive parameters by assessing follicle development, luteal function, embryo quality, and embryo development. In experiment 1 (Coscioni et al., 2003a), 27 postpubertal heifers (13 mo; 380 kg of body weight) were blocked by age and body weight (BW) and randomly assigned to one of three isocaloric and isonitrogenous diets differing only in their FG content (from cracked Pima): control (C; 0 mg of FG/kg of BW); medium (M; 20 mg of FG/kg of BW); and high (H; 40 mg of FG/kg of BW). After 30 days in the diets, the estrus was synchronized, and ovaries were scanned for follicle and corpus luteum (CL) development by ultrasound, every 24 hours, during an entire estrous cycle. Blood samples were collected daily for measurements of plasma progesterone concentrations. In experiment 2 (Coscioni et al., 2003b), 74 postpubertal heifers were blocked by age and BW and randomly assigned to one of the three diets described previously. Heifers were superovulated after 60 days in the respective diets, and embryos were collected on day 7 after the first artificial insemination. Structures were evaluated for stage of development and quality. Embryos classified as grade quality 1 to 3 were frozen and later thawed and evaluated again. Blood and uterine flushes were sampled on the day of embryo collection for determination of gossypol concentrations. In experiment 3 (Villasenor et al., 2008), 50 postpubertal Holstein heifers weighing (± SD) 406 ± 34.5 kg at 11.5 mo of age were randomly assigned to one of three isocaloric and isonitrogenous diets differing in their FG content: control (C; 0 mg of FG/kg of BW); moderate (M; 17.8 mg of FG/kg of BW); and high (H; 36.8 mg of FG/kg of BW). Heifers were fed diets for 70 days prior to superovulation and embryo collection. Superovulated heifers were flushed on day 5 after induction of ovulation, and early morulas were either stained to determine the number and proportion of live and dead cells or randomly assigned to an in vitro culture for 96 hours in media containing either 0 (CM) or 10 μg/mL (GM) of gossypol acetic acid (GAA). Plasma and uterine gossypol concentrations increased with increasing gossypol intake.
In experiment 1, emergence of first and second follicular waves (FW) were similar (P > 0.15) for C (1.1 and 9.1 d), M (1.0 and 8.9 d), and H (1.9 and 8.8 d), respectively. Deviation of the dominant follicle (DF) after emergence for the first (C = 3.5 vs. M = 3.5 vs. H = 3.4; P = 0.99) and second FW (C = 4.0 vs. M = 4.6 vs. H = 4.5; P = 0.61) was not affected by treatments. Treatment had no effect on CL growth throughout the estrous cycle (P = 0.68). Estrous cycle length (P = 0.60), maximum follicle diameter for the DF of the first and second FW (P = 0.96, P = 0.64), period of follicle dominance for the DF of the first and second FW (P = 0.99, P = 1.0), and diameter of ovulatory follicle (P = 0.36) were not influenced by dietary gossypol intake. In experiment 2, number of structures collected per heifer were, respectively, 9.4, 8.4, and 8.8 for C, M, and H (P = 0.88). Number of embryos grades 1 and 2 were similar for all treatments (P = 0.87) and averaged 3.5, 3.6, and 3.3 for C, M, and H, respectively. However, heifers receiving the H diet (5.8) had higher number (P < 0.01) of grade 3 and degenerated embryos than those receiving the C (3.6) and M (3.2) diets. Numbers of unfertilized oocytes were higher (P < 0.01) for heifers fed diets containing gossypol than for controls. High dietary gossypol retarded embryo development, and heifers fed C and M had a lower proportion of embryos classified as morula than those fed H (33.3 vs. 20.2 vs. 47.7%; P < 0.02). In experiment 3 (Villasenor et al., 2008), feeding gossypol from cottonseed increased plasma and uterine fluid concentrations of gossypol, and heifers fed H had concentrations greater than 7 μg/mL. The number of low-quality structures was greater for heifers fed H than M and C. Embryos collected from heifers fed H had the least number of cells because of fewer live cells and were smaller in diameter. High dietary gossypol reduced blastocyst development and extended the time to reach the blastocyst stage. Similarly, gossypol concentration at10 μg/mL compromised in vitro development and increased the proportion of degenerated embryos at 96 hours in culture. Results indicate that consumption of up to 40 mg of FG/kg of BW does not influence follicle and CL development in dairy heifers, but feeding a diet with 36.8 mg of FG/kg of BW increased plasma and uterine gossypol concentrations > 7 μg/mL in plasma, uterine flush, or in vitro which may compromise early embryo development, possibly explaining some of the negative effects of gossypol on fertility of dairy cows. Therefore, it is possible that reduced conception and increased pregnancy loss in dairy cattle fed high dietary gossypol previously demonstrated by our laboratory might be related to reduced embryo quality and viability.
In fact, a field study was completed to look at the effects of feeding 720 or 950 mg/kg of FG in the diet of lactating dairy cows for the first 180 DIM on reproductive and health parameters (Santos et al., 2003). Gossypol content of the diet was manipulated by partial replacement of whole Upland cottonseed with cracked Pima cottonseed. Cows consumed approximately 17 and 24 g/d of FG throughout the course of the study, which resulted in marked differences in plasma gossypol concentrations at 61 and 91 DIM (Figure 2). Type of cottonseed or dietary gossypol did not affect estrus detection rate and first service conception rate (Table 4). However, incidence of abortion increased and percentage of cows pregnant at the end of the study decreased with the high gossypol diet. These effects resulted in increased days open for cows fed the high gossypol diet (Figure 4). Looking at Figure 4, it is clear that the negative effects of gossypol on fertility only happened after 80 DIM, which probably reflects the cumulative nature of gossypol effects on reproductive events, as well as the effect of abortion on the percentage of pregnant cows at the end of the study.
| Parameter1 | Treatment | P < | |
|---|---|---|---|
| Whole Upland | Blend of whole Upland and cracked Pima |
||
| Estrus detection rate, % | 51.5 | 57.0 | 0.10 |
| CR at first AI, % | 28.2 | 29.3 | 0.73 |
| Pregnancy rate at 180 DIM, % | 79.1 | 70.6 | 0.01 |
| Abortion, % | 3.3 | 7.9 | 0.01 |
| 1CR = Conception rate, AI = artificial insemination, and DIM = days in milk. | |||

To reiterate the negative effects of gossypol on fertility of dairy cows, embryos from heifers fed 0 or 12 g/d FG for 76 days were collected, frozen, and then transferred into 269 lactating recipient cows (Galvão et al., 2006). Plasma gossypol in heifers increased with feeding cottonseed, and the concentrations were 0 and 7.38 μg/mL for heifers fed 0 and 12 g/d free gossypol, respectively. Embryos collected from heifers not fed gossypol resulted in higher pregnancy rates at 28 (33.3 vs. 23.1%) and 42 days (29.6 vs. 20.2%) of gestation compared with embryos collected from heifers fed gossypol. These data reinforced the negative effects of gossypol on fertility and, again, indicated that gossypol influences maintenance of pregnancy in cattle by changes in embryo viability.
Although increasing dietary gossypol intake by feeding Pima cottonseed had some negative effects on fertility, no health effects were observed (Table 5). Incidence of health disorders was similar between the two groups, and neither type of cottonseed or plasma gossypol influenced incidence of diseases. Similarly, culling and death rates did not differ between the two treatment groups (Table 6). Of the 13 cows that died during the course of the study, none of them had signs compatible with gossypol toxicity. Therefore, feeding diets with up to 950 mg/kg of FG for extended periods of time may affect fertility of dairy cows but seems not to affect incidence of health disorders and culling and death rates.
| Parameter | Treatment | P < | |
|---|---|---|---|
| Whole Upland | Blend of whole Upland and cracked Pima |
||
| Mastitis, % | 16.3 | 10.5 | 0.21 |
| DIM1 at first mastitis, days | 73.4 (± 7.4) | 85.5 (± 8.3) | 0.28 |
| Lameness, % | 11.1 | 16.2 | 0.57 |
| DIM at lameness diagnosis, days | 94.9 (± 10.1) | 109.4 (± 8.5) | 0.27 |
| Miscellaneous health problems, % | 7.7 | 7.8 | 0.96 |
| Any health problem, % | 30.9 | 31.6 | 0.89 |
| 1DIM = Days in milk. | |||
| Parameter | Treatment | P < | |
|---|---|---|---|
| Whole Upland | Blend of whole Upland and cracked Pima |
||
| Culling rate, % | 7.65 | 6.37 | 0.47 |
| DIM1 when culled, days | 84.4 (± 11.4) | 90.5 (± 17.2) | 0.77 |
| Mortality rate, % | 1.48 | 1.47 | 0.99 |
| DIM when dead, days | 70.0 (± 23.1) | 65.8 (± 26.8) | 0.91 |
| Left study, % | 9.14 | 7.84 | 0.51 |
| DIM when left study, days | 81.2 (± 10.2) | 79.2 (± 14.3) | 0.91 |
| 1DIM = Days in milk. | |||
Conclusions
Cottonseed is commonly fed in the diet of dairy cattle to provide protein, energy as fat, and fiber. In lactating rations, when cottonseed comprised 10% of the diet DM, increasing dietary FG concentration from 720 to 950 mg/kg of diet DM increased PG to concentrations above 7 μg/mL. Although it did not affect lactation or health of cows, it suppressed establishment and maintenance of pregnancy. Although gossypol disrupts cell metabolism and usually increases red blood cell membrane fragility, the concentrations fed in diets of dairy cows when cottonseed comprises up to 10% of the dietary DM are unlikely to result in health concerns. On the other hand, feeding free gossypol in the diet of cattle that results in PG concentrations of approximately 7 μg/mL increases gossypol concentrations in the reproductive tract, which hinders embryo development and viability, ultimately impairing fertility of cows.
Author Information
José Eduardo P. Santos
Department of Animal Sciences
University of Florida, Gainesville
References
Arieli, A. 1998. Whole cottonseed in dairy cattle feeding: A review. Anim. Feed. Sci. Technol. 72:97-110.
Brocas, C, R.M. Rivera, F.F. Paula-Lopes, L.R. McDowell, M.C. Calhoun, C.R. Staples, N.S. Wilkinson, A.J. Boning, P.J. Chenoweth, and P.J. Hansen. 1997. Deleterious actions of gossypol on bovine spermatozoa, oocytes, and embryos. Biol. Reprod. 57:901-907.
Calhoun, M.C., S.W. Kuhlmann, and B.C. Baldwin. 1995. Assessing the gossypol status of cattle fed cottonseed products. Pages 147A-157A. In Proc. Pacific Northwest Animal Nutrition Conference. Portland, OR.
Chenoweth, P.J. C.C. Chase Jr., C.A. Risco, and R.E. Larsen. 2000. Characterization of gossypol-induced sperm abnormalities in bulls. Theriogenology 53:1193-1203.
Coppock, C.E. J.K. Lanham, and J.I. Horner. 1987. A review of the nutritive value and utilization of whole cottonseed, cottonseed meal, and associated by-products by dairy cattle. Anim. Feed Sci. Technol. 18:89-129.
Coppock, C.E., J.R. Moya, J.W. West, D.H. Nave, and J.M. Labore. 1985. Effect of lint on whole cottonseed passage and digestibility and diet choice on intake of whole cottonseed by Holstein cows. J. Dairy Sci. 68:1198-1206.
Coscioni, A.C., K.N. Galvão, M. Villaseňor, J.E.P. Santos, B. Puschner, and L.M.C. Pegoraro. 2003a. Effect of gossypol intake and plasma gossypol concentrations on follicle development and luteal function in dairy heifers. J. Dairy Sci. 86(Suppl. 1):240. (Abstr.)
Coscioni, A.C., M. Villaseňor, K.N. Galvão, R. Chebe, J.E.P. Santos, J.H. Kirk, B. Puschner, and L.M.C. Pegoraro. 2003b. Effect of gossypol intake on plasma and uterine gossypol concentrations and on embryo quality and development in superovulated Holstein dairy heifers. J. Dairy Sci. 86(Suppl. 1):240 (Abstr.)
Galvão, K.N., J.E.P. Santos, A.C. Coscioni, S.O. Juchem, R.C. Chebel, W.M. Sischo, and M. Villasenor. 2006. Embryo survival from gossypol-fed heifers after transfer to lactating cows treated with human chorionic gonadotropin. J. Dairy Sci. 89:2056–2064.
Harrison, J.H., R.L. Kincaid, J.P. McNamara, S. Waltner, K.A. Loney, R.E. Riley, and J.D. Cronrath. 1995. Effect of whole cottonseed and calcium salts of long-chain fatty acids on performance of lactating dairy cows. J. Dairy Sci. 78:181-193.
Mena, H., J.E.P. Santos, J.T. Huber, J.M. Simas, M. Tarazon, and M.C. Calhoun. 2001. The effects of feeding varying amounts of gossypol from whole cottonseed and cottonseed meal in lactating dairy cows. J. Dairy Sci. 84:2231-2239.
NRC. 2001. Nutrient Requirements of Dairy Cattle. 7th rev. ed., Natl. Acad. Sci., Washington, DC.
Prieto, J.G., E.J. DePeters, P.H. Robinson, J.E.P. Santos, J.W. Pareas, S.J. Taylor, and M.C. Calhoun. 2003. Increasing dietary levels of processed Pima cottonseed increase plasma gossypol but do not influence productive performance of lactating Holstein cows. J. Dairy Sci. 86:254-267.
Randel, R.D., C.C Chase Jr., and S.J. Wyse. 1992. Effects of gossypol and cottonseed products on reproduction of mammals. J. Anim. Sci. 70:1628-1638.
Risco, C.A., C.A. Holmberg, and A. Kutches. 1992. Effect of graded concentrations of gossypol on calf performance: Toxicological and pathological considerations. J. Dairy Sci. 75:2787-2798.
Robinson, P.H., G. Getachew, E.J. DePeters, and M.C. Calhoun. 2001. Influence of variety and storage for up to 22 days on nutrient composition and gossypol level in Pima cottonseed (Gossypium spp.). Anim. Feed Sci. Technol. 91:149-156.
Santos, J.E.P., H. Mena, J.T. Huber, and M. Tarazon. 2005. Effects of source of gossypol and supplemental iron on plasma gossypol in Holstein steers. J. Dairy Sci. 88:3563-3574
Santos, J.E.P., M. Villaseňor, E.J. DePeters, P.H. Robinson, and B.C. Baldwin. 2002. Type of cottonseed and gossypol in diets of lactating dairy cows: Lactation performance and plasma gossypol. J. Dairy Sci. 85:1491-1501.
Santos, J.E.P., M. Villaseňor, E.J. DePeters, P.H. Robinson, and C.H. Holmberg. 2003. Type of cottonseed and gossypol in diets of lactating dairy cows: Plasma gossypol, reproduction, and health. J. Dairy Sci. 86:892-905.
Smith, W.A., B. Harris, H.H. van Horn, and C.J. Wilcox. 1993. Effect of forage type on production of dairy cows supplemented with whole cottonseed, tallow, and yeast. J. Dairy Sci. 76:205-215.
Sullivan, J.L., J.T. Huber, and J.M. Harper. 1993a. Performance of dairy cows fed short staple, Pima, and cracked Pima cottonseed and feed characteristics. J. Dairy Sci. 76:3555-3561.
Sullivan, J.L., J.T. Huber, R.L. Price, and J.M. Harper. 1993b. Comparison of digestibility, nutritive value, and storage characteristics of different forms of cottonseed in diets fed to lactating dairy cows. J. Anim. Sci. 71:2837-2842.
Velasquez-Pereira, J., P.J. Chenoweth, L.R. McDowell, C.A. Risco, C.A. Staples, D. Prichard, F.G. Martin, M.C. Calhoun, S.N. Williams, and N.S. Wilkinson. 1998. Reproductive effects of feeding gossypol and vitamin E to bulls. J Anim. Sci. 76:2894-2904.
Villaseňor, M., A.C. Coscioni, K.N. Galvão, R.C. Chebel, and J.E.P. Santos. 2008. Gossypol disrupts embryo development in heifers. J. Dairy Sci. 91:3015-3024.
Zinn, R.A. 1995. Characteristics of digestion of linted and lint-free cottonseed in diets for feedlot cattle. J. Anim. Sci. 73:1246-1250.
